Silencing mutant ATXN3 expression resolves molecular phenotypes in SCA3 transgenic mice
- PMID: 23820820
- PMCID: PMC3808130
- DOI: 10.1038/mt.2013.152
Silencing mutant ATXN3 expression resolves molecular phenotypes in SCA3 transgenic mice
Erratum in
- Mol Ther. 2014 Apr;22(4):891. Costa, Maria doCarmo [corrected to Costa, Maria do Carmo]
Abstract
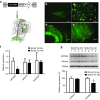
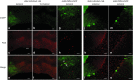
Spinocerebellar ataxia type 3 (SCA3) is a neurodegenerative disease caused by a polyglutamine expansion in the deubiquitinating enzyme, Ataxin-3. Currently, there are no effective treatments for this fatal disorder but studies support the hypothesis that reducing mutant Ataxin-3 protein levels might reverse or halt the progression of disease in SCA3. Here, we sought to modulate ATXN3 expression in vivo using RNA interference. We developed artificial microRNA mimics targeting the 3'-untranslated region (3'UTR) of human ATXN3 and then used recombinant adeno-associated virus to deliver them to the cerebellum of transgenic mice expressing the full human disease gene (SCA3/MJD84.2 mice). Anti-ATXN3 microRNA mimics effectively suppressed human ATXN3 expression in SCA3/MJD84.2 mice. Short-term treatment cleared the abnormal nuclear accumulation of mutant Ataxin-3 throughout the transduced SCA3/MJD84.2 cerebellum. Analysis also revealed changes in the steady-state levels of specific microRNAs in the cerebellum of SCA3/MJD84.2 mice, a previously uncharacterized molecular phenotype of SCA3 that appears to be dependent on mutant Ataxin-3 expression. Our findings support the preclinical development of molecular therapies aimed at halting the expression of ATXN3 as a viable approach to SCA3 and point to microRNA deregulation as a potential surrogate marker of SCA3 pathogenesis.
Figures


References
-
- Matos CA, de Macedo-Ribeiro S, Carvalho AL. Polyglutamine diseases: the special case of ataxin-3 and Machado-Joseph disease. Prog Neurobiol. 2011;95:26–48. - PubMed
-
- Durr A. Autosomal dominant cerebellar ataxias: polyglutamine expansions and beyond. Lancet Neurol. 2010;9:885–894. - PubMed
-
- Boy J, Schmidt T, Wolburg H, Mack A, Nuber S, Böttcher M, et al. Reversibility of symptoms in a conditional mouse model of spinocerebellar ataxia type 3. Hum Mol Genet. 2009;18:4282–4295. - PubMed
-
- Yamamoto A, Lucas JJ, Hen R. Reversal of neuropathology and motor dysfunction in a conditional model of Huntington's disease. Cell. 2000;101:57–66. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

