Minireview: Novel aspects of M3 muscarinic receptor signaling in pancreatic β-cells
- PMID: 23820900
- PMCID: PMC3725345
- DOI: 10.1210/me.2013-1084
Minireview: Novel aspects of M3 muscarinic receptor signaling in pancreatic β-cells
Abstract
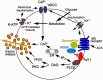
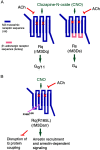
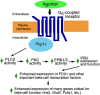
The release of insulin from pancreatic β-cells is regulated by a considerable number of G protein-coupled receptors. During the past several years, we have focused on the physiological importance of β-cell M3 muscarinic acetylcholine receptors (M3Rs). At the molecular level, the M3R selectively activates G proteins of the G(q) family. Phenotypic analysis of several M3R mutant mouse models, including a mouse strain that lacks M3Rs only in pancreatic β-cells, indicated that β-cell M3Rs play a key role in maintaining blood glucose levels within a normal range. Additional studies with transgenic M3R mouse models strongly suggest that strategies aimed to enhance signaling through β-cell M3Rs may prove useful in the treatment of type 2 diabetes. More recently, we analyzed transgenic mice that expressed an M3R-based designer receptor in a β-cell-specific fashion, which enabled us to chronically activate a β-cell G(q)-coupled receptor by a drug that is otherwise pharmacologically inert. Drug-dependent activation of this designer receptor stimulated the sequential activation of G(q), phospholipase C, ERK1/2, and insulin receptor substrate 2 signaling, thus triggering a series of events that greatly improved β-cell function. Most importantly, chronic stimulation of this pathway protected mice against experimentally induced diabetes and glucose intolerance, induced either by streptozotocin or by the consumption of an energy-rich, high-fat diet. Because β-cells are endowed with numerous receptors that mediate their cellular effects via activation of G(q)-type G proteins, these findings provide a rational basis for the development of novel antidiabetic drugs targeting this class of receptors.
Figures
Similar articles
-
Allosteric modulation of β-cell M3 muscarinic acetylcholine receptors greatly improves glucose homeostasis in lean and obese mice.Proc Natl Acad Sci U S A. 2019 Sep 10;116(37):18684-18690. doi: 10.1073/pnas.1904943116. Epub 2019 Aug 26. Proc Natl Acad Sci U S A. 2019. PMID: 31451647 Free PMC article.
-
Chronic activation of a designer G(q)-coupled receptor improves β cell function.J Clin Invest. 2013 Apr;123(4):1750-62. doi: 10.1172/JCI66432. Epub 2013 Mar 8. J Clin Invest. 2013. PMID: 23478411 Free PMC article.
-
Beneficial metabolic effects caused by persistent activation of beta-cell M3 muscarinic acetylcholine receptors in transgenic mice.Endocrinology. 2010 Nov;151(11):5185-94. doi: 10.1210/en.2010-0519. Epub 2010 Sep 15. Endocrinology. 2010. PMID: 20843999 Free PMC article.
-
Novel insights into the function of β-cell M3 muscarinic acetylcholine receptors: therapeutic implications.Trends Endocrinol Metab. 2011 Feb;22(2):74-80. doi: 10.1016/j.tem.2010.10.004. Epub 2010 Nov 23. Trends Endocrinol Metab. 2011. PMID: 21106385 Free PMC article. Review.
-
Beta-cell M3 muscarinic acetylcholine receptors as potential targets for novel antidiabetic drugs.Int Immunopharmacol. 2020 Apr;81:106267. doi: 10.1016/j.intimp.2020.106267. Epub 2020 Feb 7. Int Immunopharmacol. 2020. PMID: 32044662 Review.
Cited by
-
Inhibition of cholinergic potentiation of insulin secretion from pancreatic islets by chronic elevation of glucose and fatty acids: Protection by casein kinase 2 inhibitor.Mol Metab. 2017 Oct;6(10):1240-1253. doi: 10.1016/j.molmet.2017.07.017. Epub 2017 Aug 4. Mol Metab. 2017. PMID: 29031723 Free PMC article.
-
Contribution of parasympathetic muscarinic augmentation of insulin secretion to olanzapine-induced hyperinsulinemia.Am J Physiol Endocrinol Metab. 2018 Aug 1;315(2):E250-E257. doi: 10.1152/ajpendo.00315.2017. Epub 2017 Dec 19. Am J Physiol Endocrinol Metab. 2018. PMID: 29351487 Free PMC article. Clinical Trial.
-
Minireview: Role of intracellular scaffolding proteins in the regulation of endocrine G protein-coupled receptor signaling.Mol Endocrinol. 2015 Jun;29(6):814-30. doi: 10.1210/me.2015-1091. Epub 2015 May 5. Mol Endocrinol. 2015. PMID: 25942107 Free PMC article. Review.
-
Role of Serotonin Transporter in Antidepressant-Induced Diabetes Mellitus: A Pharmacoepidemiological-Pharmacodynamic Study in VigiBase®.Drug Saf. 2018 Nov;41(11):1087-1096. doi: 10.1007/s40264-018-0693-8. Drug Saf. 2018. PMID: 29956218
-
Incretin-Based Therapies: Revisiting Their Mode of Action.Endocrinology. 2017 Jun 1;158(6):1560-1563. doi: 10.1210/en.2017-00252. Endocrinology. 2017. PMID: 28575434 Free PMC article. No abstract available.
References
-
- Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846 - PubMed
-
- Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. 2005;365:1333–1346 - PubMed
-
- Ahrén B. Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nat Rev Drug Discov. 2009;8:369–385 - PubMed
-
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

