Bioengineered nanoparticles for siRNA delivery
- PMID: 23821336
- PMCID: PMC3972625
- DOI: 10.1002/wnan.1233
Bioengineered nanoparticles for siRNA delivery
Abstract
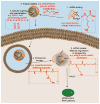
Short interfering RNA (siRNA) has been an important laboratory tool in the last two decades and has allowed researchers to better understand the functions of nonprotein-coding genes through RNA interference (RNAi). Although RNAi holds great promise for this purpose as well as for treatment of many diseases, efforts at using siRNA have been hampered by the difficulty of safely and effectively introducing it into cells of interest, both in vitro and in vivo. To overcome this challenge, many biomaterials and nanoparticles (NPs) have been developed and optimized for siRNA delivery, often taking cues from the DNA delivery field, although different barriers exist for these two types of molecules. In this review, we discuss general properties of biomaterials and nanoparticles that are necessary for effective nucleic acid delivery. We also discuss specific examples of bioengineered materials, including lipid-based NPs, polymeric NPs, inorganic NPs, and RNA-based NPs, which clearly illustrate the problems and successes in siRNA delivery.
© 2013 Wiley Periodicals, Inc.
Figures


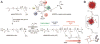

Similar articles
-
Nanoparticles containing insoluble drug for cancer therapy.Biotechnol Adv. 2014 Jul-Aug;32(4):778-88. doi: 10.1016/j.biotechadv.2013.10.002. Epub 2013 Oct 8. Biotechnol Adv. 2014. PMID: 24113214 Free PMC article. Review.
-
Recent advances in nanoparticle-mediated siRNA delivery.Annu Rev Biomed Eng. 2014 Jul 11;16:347-70. doi: 10.1146/annurev-bioeng-071813-105119. Epub 2014 Jun 2. Annu Rev Biomed Eng. 2014. PMID: 24905873 Review.
-
Rigid nanoparticle-based delivery of anti-cancer siRNA: challenges and opportunities.Biotechnol Adv. 2014 Jul-Aug;32(4):831-43. doi: 10.1016/j.biotechadv.2013.08.020. Epub 2013 Sep 5. Biotechnol Adv. 2014. PMID: 24013011 Free PMC article. Review.
-
[Types of non-viral delivery systems of small interfering RNA].Mol Biol (Mosk). 2012 May-Jun;46(3):387-401. Mol Biol (Mosk). 2012. PMID: 22888629 Review. Russian.
-
Biomaterials in siRNA Delivery: A Comprehensive Review.Adv Healthc Mater. 2016 Nov;5(21):2715-2731. doi: 10.1002/adhm.201600418. Epub 2016 Oct 4. Adv Healthc Mater. 2016. PMID: 27700013 Review.
Cited by
-
Engineering oncogene-targeted anisamide-functionalized pBAE nanoparticles as efficient lung cancer antisense therapies.RSC Adv. 2023 Oct 13;13(43):29986-30001. doi: 10.1039/d3ra05830a. eCollection 2023 Oct 11. RSC Adv. 2023. PMID: 37842686 Free PMC article.
-
Exogenous dsRNA-Mediated RNAi: Mechanisms, Applications, Delivery Methods and Challenges in the Induction of Viral Disease Resistance in Plants.Viruses. 2024 Dec 31;17(1):49. doi: 10.3390/v17010049. Viruses. 2024. PMID: 39861836 Free PMC article. Review.
-
Epigenetic therapy targeting bone marrow mesenchymal stem cells for age-related bone diseases.Stem Cell Res Ther. 2022 May 16;13(1):201. doi: 10.1186/s13287-022-02852-w. Stem Cell Res Ther. 2022. PMID: 35578312 Free PMC article. Review.
-
Joining Forces: The Combined Application of Therapeutic Viruses and Nanomaterials in Cancer Therapy.Molecules. 2023 Nov 20;28(22):7679. doi: 10.3390/molecules28227679. Molecules. 2023. PMID: 38005401 Free PMC article. Review.
-
Cyclic Peptide-Gadolinium Nanocomplexes as siRNA Delivery Tools.Pharmaceuticals (Basel). 2021 Oct 20;14(11):1064. doi: 10.3390/ph14111064. Pharmaceuticals (Basel). 2021. PMID: 34832846 Free PMC article.
References
-
- Fire A, Xu SQ, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. - PubMed
-
- Baulcombe D. RNA silencing in plants. Nature. 2004;431:356–363. - PubMed
-
- Sanchez-Vargas I, Travanty EA, Keene KM, Franz AWE, Beaty BJ, Blair CD, Olson KE. RNA interference, arthropod-borne viruses, and mosquitoes. Virus Res. 2004;102:65–74. - PubMed
-
- Voinnet O. Induction and suppression of RNA silencing: insights from viral infections. Nat Rev Genet. 2005;6:206–220. - PubMed
-
- Kuwabara PE, Coulson A. RNAi--prospects for a general technique for determining gene function. Parasitol Today. 2000;16:347–349. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

