Host Organelle Hijackers: a similar modus operandi for Toxoplasma gondii and Chlamydia trachomatis: co-infection model as a tool to investigate pathogenesis
- PMID: 23821471
- PMCID: PMC3808458
- DOI: 10.1111/2049-632X.12057
Host Organelle Hijackers: a similar modus operandi for Toxoplasma gondii and Chlamydia trachomatis: co-infection model as a tool to investigate pathogenesis
Abstract
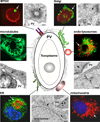
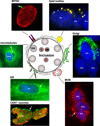
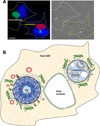
The bacterium Chlamydia trachomatis and the protozoan parasite Toxoplasma gondii are the causative agents of chlamydiosis and toxoplasmosis in humans, respectively. Both microorganisms are obligate intracellular pathogens and notorious for extensively modifying the cytoskeletal architecture and the endomembrane system of their host cells to establish productive infections. This review highlights the similar tactics developed by these two pathogens to manipulate their host cell despite their genetic unrelatedness. Using an in vitro cell culture model whereby single fibroblasts are infected by C. trachomatis and T. gondii simultaneously, thus setting up an intracellular competition, we demonstrate that the solutions to the problem of intracellular survival deployed by the parasite and the bacterium may represent an example of convergent evolution, driven by the necessity to acquire nutrients in a hostile environment.
Keywords: bacteria; convergent evolution; host organelle interaction; intracellular parasitism; nutrient scavenging; protozoa.
© 2013 Federation of European Microbiological Societies. Published by John Wiley & Sons Ltd. All rights reserved.
Figures
Similar articles
-
Fierce competition between Toxoplasma and Chlamydia for host cell structures in dually infected cells.Eukaryot Cell. 2013 Feb;12(2):265-77. doi: 10.1128/EC.00313-12. Epub 2012 Dec 14. Eukaryot Cell. 2013. PMID: 23243063 Free PMC article.
-
A novel co-infection model with Toxoplasma and Chlamydia trachomatis highlights the importance of host cell manipulation for nutrient scavenging.Cell Microbiol. 2013 Apr;15(4):619-46. doi: 10.1111/cmi.12060. Epub 2012 Nov 27. Cell Microbiol. 2013. PMID: 23107293 Free PMC article.
-
Association between intracellular infectious agents and Tourette's syndrome.Eur Arch Psychiatry Clin Neurosci. 2010 Jun;260(4):359-63. doi: 10.1007/s00406-009-0084-3. Epub 2009 Nov 5. Eur Arch Psychiatry Clin Neurosci. 2010. PMID: 19890596
-
Kiss and spit: the dual roles of Toxoplasma rhoptries.Nat Rev Microbiol. 2008 Jan;6(1):79-88. doi: 10.1038/nrmicro1800. Nat Rev Microbiol. 2008. PMID: 18059289 Review.
-
Apoptosis and its modulation during infection with Toxoplasma gondii: molecular mechanisms and role in pathogenesis.Curr Top Microbiol Immunol. 2005;289:219-37. doi: 10.1007/3-540-27320-4_10. Curr Top Microbiol Immunol. 2005. PMID: 15791958 Review.
Cited by
-
Transcriptomic and metabolomic analyses reveal the essential nature of Rab1B in Toxoplasma gondii.Parasit Vectors. 2023 Nov 8;16(1):409. doi: 10.1186/s13071-023-06030-6. Parasit Vectors. 2023. PMID: 37941035 Free PMC article.
-
The critical role of Toxoplasma gondii GRA1 in nutrient salvage.mBio. 2025 Aug 13;16(8):e0124225. doi: 10.1128/mbio.01242-25. Epub 2025 Jun 27. mBio. 2025. PMID: 40576341 Free PMC article.
-
Toxoplasma Mechanisms for Delivery of Proteins and Uptake of Nutrients Across the Host-Pathogen Interface.Annu Rev Microbiol. 2020 Sep 8;74:567-586. doi: 10.1146/annurev-micro-011720-122318. Epub 2020 Jul 17. Annu Rev Microbiol. 2020. PMID: 32680452 Free PMC article. Review.
-
Modelling Toxoplasma gondii infection in a 3D cell culture system In Vitro: Comparison with infection in 2D cell monolayers.PLoS One. 2018 Dec 6;13(12):e0208558. doi: 10.1371/journal.pone.0208558. eCollection 2018. PLoS One. 2018. PMID: 30521607 Free PMC article.
-
Hostile intruder: Toxoplasma holds host organelles captive.PLoS Pathog. 2018 Mar 29;14(3):e1006893. doi: 10.1371/journal.ppat.1006893. eCollection 2018 Mar. PLoS Pathog. 2018. PMID: 29596535 Free PMC article. No abstract available.
References
-
- Alvarez M, Casadevall A. Phagosome extrusion and host-cell survival after Cryptococcus neoformans phagocytosis by macrophages. Curr Biol. 2006;16:2161–2165. - PubMed
-
- Andersson SG, Kurland CG. Reductive evolution of resident genomes. Trends Microbiol. 1998;6:263–268. - PubMed
-
- Antoine JC, Prina E, Lang T, Courret N. The biogenesis and properties of the parasitophorous vacuoles that harbour Leishmania in murine macrophages. Trends Microbiol. 1998;6:392–401. - PubMed
-
- Anttila T, Saikku P, Koskela P, et al. Serotypes of Chlamydia trachomatis and risk for development of cervical squamous cell carcinoma. JAMA. 2001;285:47–51. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

