The Legionella pneumophila GTPase activating protein LepB accelerates Rab1 deactivation by a non-canonical hydrolytic mechanism
- PMID: 23821544
- PMCID: PMC3745345
- DOI: 10.1074/jbc.M113.470625
The Legionella pneumophila GTPase activating protein LepB accelerates Rab1 deactivation by a non-canonical hydrolytic mechanism
Abstract
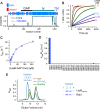
GTPase activating proteins (GAPs) from pathogenic bacteria and eukaryotic host organisms deactivate Rab GTPases by supplying catalytic arginine and glutamine fingers in trans and utilizing the cis-glutamine in the DXXGQ motif of the GTPase for binding rather than catalysis. Here, we report the transition state mimetic structure of the Legionella pneumophila GAP LepB in complex with Rab1 and describe a comprehensive structure-based mutational analysis of potential catalytic and recognition determinants. The results demonstrate that LepB does not simply mimic other GAPs but instead deploys an expected arginine finger in conjunction with a novel glutamic acid finger, which forms a salt bridge with an indispensible switch II arginine that effectively locks the cis-glutamine in the DXXGQ motif of Rab1 in a catalytically competent though unprecedented transition state configuration. Surprisingly, a heretofore universal transition state interaction with the cis-glutamine is supplanted by an elaborate polar network involving critical P-loop and switch I serines. LepB further employs an unusual tandem domain architecture to clamp a switch I tyrosine in an open conformation that facilitates access of the arginine finger to the hydrolytic site. Intriguingly, the critical P-loop serine corresponds to an oncogenic substitution in Ras and replaces a conserved glycine essential for the canonical transition state stereochemistry. In addition to expanding GTP hydrolytic paradigms, these observations reveal the unconventional dual finger and non-canonical catalytic network mechanisms of Rab GAPs as necessary alternative solutions to a major impediment imposed by substitution of the conserved P-loop glycine.
Keywords: GAP; GTPase; GTPase Activating Protein; Host-Pathogen Interactions; Legionella pneumophila; LepB; Rab; Rab Proteins; Rab1; X-ray Crystallography.
Figures





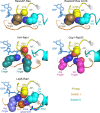
Similar articles
-
TBC-domain GAPs for Rab GTPases accelerate GTP hydrolysis by a dual-finger mechanism.Nature. 2006 Jul 20;442(7100):303-6. doi: 10.1038/nature04847. Nature. 2006. PMID: 16855591
-
Structural analyses of Legionella LepB reveal a new GAP fold that catalytically mimics eukaryotic RasGAP.Cell Res. 2013 Jun;23(6):775-87. doi: 10.1038/cr.2013.54. Epub 2013 Apr 16. Cell Res. 2013. PMID: 23588383 Free PMC article.
-
Mechanism of Rab1b deactivation by the Legionella pneumophila GAP LepB.EMBO Rep. 2013 Feb;14(2):199-205. doi: 10.1038/embor.2012.211. Epub 2013 Jan 4. EMBO Rep. 2013. PMID: 23288104 Free PMC article.
-
Common mechanisms of catalysis in small and heterotrimeric GTPases and their respective GAPs.Biol Chem. 2017 May 1;398(5-6):523-533. doi: 10.1515/hsz-2016-0314. Biol Chem. 2017. PMID: 28245182 Review.
-
De-AMPylation unmasked: modulation of host membrane trafficking.Sci Signal. 2011 Oct 11;4(194):pe42. doi: 10.1126/scisignal.2002458. Sci Signal. 2011. PMID: 21990428 Review.
Cited by
-
Invited review: Small GTPases and their GAPs.Biopolymers. 2016 Aug;105(8):431-48. doi: 10.1002/bip.22833. Biopolymers. 2016. PMID: 26972107 Free PMC article. Review.
-
Genomic analysis of 38 Legionella species identifies large and diverse effector repertoires.Nat Genet. 2016 Feb;48(2):167-75. doi: 10.1038/ng.3481. Epub 2016 Jan 11. Nat Genet. 2016. PMID: 26752266 Free PMC article.
-
Phosphoinositides and the Fate of Legionella in Phagocytes.Front Immunol. 2020 Jan 30;11:25. doi: 10.3389/fimmu.2020.00025. eCollection 2020. Front Immunol. 2020. PMID: 32117224 Free PMC article. Review.
-
The unity of opposites: Strategic interplay between bacterial effectors to regulate cellular homeostasis.J Biol Chem. 2021 Dec;297(6):101340. doi: 10.1016/j.jbc.2021.101340. Epub 2021 Oct 23. J Biol Chem. 2021. PMID: 34695417 Free PMC article. Review.
-
Study of Legionella Effector Domains Revealed Novel and Prevalent Phosphatidylinositol 3-Phosphate Binding Domains.Infect Immun. 2019 May 21;87(6):e00153-19. doi: 10.1128/IAI.00153-19. Print 2019 Jun. Infect Immun. 2019. PMID: 30962397 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

