Severe everolimus-induced steatohepatis: a case report
- PMID: 23822543
- PMCID: PMC3706391
- DOI: 10.1186/2047-783X-18-22
Severe everolimus-induced steatohepatis: a case report
Abstract
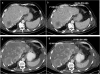
The mammalian target of rapamycin inhibitors are normally favored as immunosuppressant agents for solid organ transplantation such as kidney, liver or heart. Only in recent years have they been increasingly administered for the treatment of neuroendocrine tumors. Even though mammalian target of rapamycin inhibitors are known to exhibit specific side effects, everolimus-related severe hepatic steatosis has not as yet been described in the literature. We report the case of a 76-year-old man who developed severe hepatic steatosis within four weeks of treatment with everolimus as concomitant tumor therapy for a progressively growing neuroendocrine carcinoma of the ileum. A diagnosis of hepatic steatosis was established using computer tomography and fibroscan©. Other underlying causes for steatosis hepatis could be excluded. Further studies are warranted to explain the underlying mechanisms.
Figures
References
-
- Bolke E, Schieren G, Gripp S, Steinbach G, Peiper M, Orth K, Matuschek C, Pelzer M, Lammering G, Houben R, Antke C, Rump LC, Mota R, Gerber PA, Schuler P, Hoffmann TK, Rusnak E, Hermsen D, Budach W. Cystatin C - a fast and reliable biomarker for glomerular filtration rate in head and neck cancer patients. Strahlenther Onkol. 2011;187:191–201. doi: 10.1007/s00066-010-2203-5. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

