PTHrP Overexpression Increases Sensitivity of Breast Cancer Cells to Apo2L/TRAIL
- PMID: 23822995
- PMCID: PMC3688876
- DOI: 10.1371/journal.pone.0066343
PTHrP Overexpression Increases Sensitivity of Breast Cancer Cells to Apo2L/TRAIL
Abstract
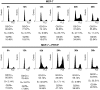
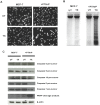
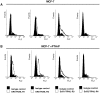
Parathyroid hormone-related protein (PTHrP) is a key component in breast development and breast tumour biology. PTHrP has been discovered as a causative agent of hypercalcaemia of malignancy and is also one of the main factors implicated in breast cancer mediated osteolysis. Clinical studies have determined that PTHrP expression by primary breast cancers was an independent predictor of improved prognosis. Furthermore, PTHrP has been demonstrated to cause tumour cell death both in vitro and in vivo. Apo2L/TRAIL is a promising new anti-cancer agent, due to its ability to selectively induce apoptosis in cancer cells whilst sparing most normal cells. However, some cancer cells are resistant to Apo2L/TRAIL-induced apoptosis thus limiting its therapeutic efficacy. The effects of PTHrP on cell death signalling pathways initiated by Apo2L/TRAIL were investigated in breast cancer cells. Expression of PTHrP in Apo2L/TRAIL resistant cell line MCF-7 sensitised these cells to Apo2L/TRAIL-induced apoptosis. The actions of PTHrP resulted from intracellular effects, since exogenous treatment of PTHrP had no effect on Apo2L/TRAIL-induced apoptosis. Apo2L/TRAIL-induced apoptosis in PTHrP expressing cells occurred through the activation of caspase-10 resulting in caspase-9 activation and induction of apoptosis through the effector caspases, caspase-6 and -7. PTHrP increased cell surface expression of Apo2L/TRAIL death receptors, TRAIL-R1 and TRAIL-R2. Antagonistic antibodies against the death receptors demonstrated that Apo2L/TRAIL mediated its apoptotic signals through activation of the TRAIL-R2 in PTHrP expressing breast cancer cells. These studies reveal a novel role for PTHrP with Apo2L/TRAIL that maybe important for future diagnosis and treatment of breast cancer.
Conflict of interest statement
Figures




Similar articles
-
The histone deacetylase inhibitor, suberoylanilide hydroxamic acid, overcomes resistance of human breast cancer cells to Apo2L/TRAIL.Int J Cancer. 2006 Aug 15;119(4):944-54. doi: 10.1002/ijc.21939. Int J Cancer. 2006. PMID: 16550602
-
The essential role of the mitochondria-dependent death-signaling cascade in chemotherapy-induced potentiation of Apo2L/TRAIL cytotoxicity in cultured thoracic cancer cells: amplified caspase 8 is indispensable for combination-mediated massive cell death.Cancer J. 2006 Jul-Aug;12(4):257-73. doi: 10.1097/00130404-200607000-00004. Cancer J. 2006. PMID: 16925970
-
Sensitization of tumor cells to Apo2 ligand/TRAIL-induced apoptosis by inhibition of casein kinase II.Cancer Res. 2002 Aug 1;62(15):4180-5. Cancer Res. 2002. PMID: 12154014
-
Targeting the Apo2L/TRAIL system for the therapy of autoimmune diseases and cancer.Biochem Pharmacol. 2012 Jun 1;83(11):1475-83. doi: 10.1016/j.bcp.2011.12.036. Epub 2012 Jan 2. Biochem Pharmacol. 2012. PMID: 22230480 Review.
-
Down-regulation of intracellular anti-apoptotic proteins, particularly c-FLIP by therapeutic agents; the novel view to overcome resistance to TRAIL.J Cell Physiol. 2018 Oct;233(10):6470-6485. doi: 10.1002/jcp.26585. Epub 2018 May 9. J Cell Physiol. 2018. PMID: 29741767 Review.
Cited by
-
Metformin prevention of doxorubicin resistance in MCF-7 and MDA-MB-231 involves oxidative stress generation and modulation of cell adaptation genes.Sci Rep. 2019 Apr 10;9(1):5864. doi: 10.1038/s41598-019-42357-w. Sci Rep. 2019. PMID: 30971831 Free PMC article.
-
Parathyroid hormone-related protein overexpression protects goat mammary gland epithelial cells from calcium-sensing receptor activation-induced apoptosis.Mol Biol Rep. 2015 Jan;42(1):233-43. doi: 10.1007/s11033-014-3763-8. Epub 2014 Sep 30. Mol Biol Rep. 2015. PMID: 25266236
-
Calcium-Sensing Receptor Promotes Breast Cancer by Stimulating Intracrine Actions of Parathyroid Hormone-Related Protein.Cancer Res. 2016 Sep 15;76(18):5348-60. doi: 10.1158/0008-5472.CAN-15-2614. Epub 2016 Jul 22. Cancer Res. 2016. PMID: 27450451 Free PMC article.
References
-
- Winter MC, Coleman RE (2009) Bisphosphonates in breast cancer: Teaching an old dog new tricks. Current Opinion in Oncology 21: 499–506. - PubMed
-
- Martin TJ, Moseley JM (2000) Mechanisms in the skeletal complications of breast cancer. Endocr Relat Cancer 7: 271–284. - PubMed
-
- Suva LJ, Winslow GA, Wettenhall RE, Hammonds RG, Moseley JM, et al. (1987) A parathyroid hormone-related protein implicated in malignant hypercalcemia: cloning and expression. Science 237: 893–896. - PubMed
-
- Henderson MA, Danks JA, Slavin JL, Byrnes GB, Choong PF, et al. (2006) Parathyroid hormone-related protein localization in breast cancers predict improved prognosis. Cancer Res 66: 2250–2256. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

