Sensorimotor and Pain Modulation Brain Abnormalities in Trigeminal Neuralgia: A Paroxysmal, Sensory-Triggered Neuropathic Pain
- PMID: 23823184
- PMCID: PMC3688879
- DOI: 10.1371/journal.pone.0066340
Sensorimotor and Pain Modulation Brain Abnormalities in Trigeminal Neuralgia: A Paroxysmal, Sensory-Triggered Neuropathic Pain
Abstract
Objective: Idiopathic trigeminal neuralgia (TN) is characterized by paroxysms of severe facial pain but without the major sensory loss that commonly accompanies neuropathic pain. Since neurovascular compression of the trigeminal nerve root entry zone does not fully explain the pathogenesis of TN, we determined whether there were brain gray matter abnormalities in a cohort of idiopathic TN patients. We used structural MRI to test the hypothesis that TN is associated with altered gray matter (GM) in brain areas involved in the sensory and affective aspects of pain, pain modulation, and motor function. We further determined the contribution of long-term TN on GM plasticity.
Methods: Cortical thickness and subcortical GM volume were measured from high-resolution 3T T1-weighted MRI scans in 24 patients with right-sided TN and 24 healthy control participants.
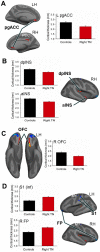
Results: TN patients had increased GM volume in the sensory thalamus, amygdala, periaqueductal gray, and basal ganglia (putamen, caudate, nucleus accumbens) compared to healthy controls. The patients also had greater cortical thickness in the contralateral primary somatosensory cortex and frontal pole compared to controls. In contrast, patients had thinner cortex in the pregenual anterior cingulate cortex, the insula and the orbitofrontal cortex. No relationship was observed between GM abnormalities and TN pain duration.
Conclusions: TN is associated with GM abnormalities in areas involved in pain perception, pain modulation and motor function. These findings may reflect increased nociceptive input to the brain, an impaired descending modulation system that does not adequately inhibit pain, and increased motor output to control facial movements to limit pain attacks.
Conflict of interest statement
Figures


Similar articles
-
Reversal of insular and microstructural nerve abnormalities following effective surgical treatment for trigeminal neuralgia.Pain. 2015 Jun;156(6):1112-1123. doi: 10.1097/j.pain.0000000000000156. Pain. 2015. PMID: 25782366
-
Altered structure and functional connection in patients with classical trigeminal neuralgia.Hum Brain Mapp. 2018 Feb;39(2):609-621. doi: 10.1002/hbm.23696. Epub 2017 Nov 6. Hum Brain Mapp. 2018. PMID: 29105886 Free PMC article.
-
Gray matter volume reduction reflects chronic pain in trigeminal neuralgia.Neuroimage. 2013 Jul 1;74:352-8. doi: 10.1016/j.neuroimage.2013.02.029. Epub 2013 Feb 26. Neuroimage. 2013. PMID: 23485849
-
Grey matter volume alterations in trigeminal neuralgia: A systematic review and meta-analysis of voxel-based morphometry studies.Prog Neuropsychopharmacol Biol Psychiatry. 2020 Mar 2;98:109821. doi: 10.1016/j.pnpbp.2019.109821. Epub 2019 Nov 19. Prog Neuropsychopharmacol Biol Psychiatry. 2020. PMID: 31756417
-
Alterations in grey matter density and functional connectivity in trigeminal neuropathic pain and trigeminal neuralgia: A systematic review and meta-analysis.Neuroimage Clin. 2019;24:102039. doi: 10.1016/j.nicl.2019.102039. Epub 2019 Oct 17. Neuroimage Clin. 2019. PMID: 31698316 Free PMC article.
Cited by
-
Shedding light on neuropathic pain: Current and emerging tools for diagnosis, screening, and quantification.SAGE Open Med. 2024 Feb 9;12:20503121231218985. doi: 10.1177/20503121231218985. eCollection 2024. SAGE Open Med. 2024. PMID: 38343869 Free PMC article. Review.
-
White Matter Diffusion Properties in Chronic Temporomandibular Disorders: An Exploratory Analysis.Front Pain Res (Lausanne). 2022 Jun 21;3:880831. doi: 10.3389/fpain.2022.880831. eCollection 2022. Front Pain Res (Lausanne). 2022. PMID: 35800990 Free PMC article.
-
Widespread Volumetric Brain Changes following Tooth Loss in Female Mice.Front Neuroanat. 2017 Jan 9;10:121. doi: 10.3389/fnana.2016.00121. eCollection 2016. Front Neuroanat. 2017. PMID: 28119577 Free PMC article.
-
Magnetic resonance imaging for chronic pain: diagnosis, manipulation, and biomarkers.Sci China Life Sci. 2021 Jun;64(6):879-896. doi: 10.1007/s11427-020-1822-4. Epub 2020 Nov 23. Sci China Life Sci. 2021. PMID: 33247802 Review.
-
Altered functional activity and connectivity in Parkinson's disease with chronic pain: a resting-state fMRI study.Front Aging Neurosci. 2025 Mar 3;17:1499262. doi: 10.3389/fnagi.2025.1499262. eCollection 2025. Front Aging Neurosci. 2025. PMID: 40099248 Free PMC article.
References
-
- Maier C, Baron R, Tolle TR, Binder A, Birbaumer N, et al. (2010) Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. Pain 150: 439–450. - PubMed
-
- Sinay VJ, Bonamico LH, Dubrovsky A (2003) Subclinical sensory abnormalities in trigeminal neuralgia. Cephalalgia 23: 541–544. - PubMed
-
- Siccoli MM, Bassetti CL, Sandor PS (2006) Facial pain: clinical differential diagnosis. Lancet Neurol 5: 257–267. - PubMed
-
- Siddiqui MN, Siddiqui S, Ranasinghe JS, Furgang FA (2003) Pain management: trigeminal neuralgia. Hospital Physician 39: 64–70.
-
- Rappaport ZH, Devor M (1994) Trigeminal neuralgia: the role of self-sustaining discharge in the trigeminal ganglion. Pain 56: 127–138. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

