Nonlinear strain stiffening is not sufficient to explain how far cells can feel on fibrous protein gels
- PMID: 23823219
- PMCID: PMC3699756
- DOI: 10.1016/j.bpj.2013.05.032
Nonlinear strain stiffening is not sufficient to explain how far cells can feel on fibrous protein gels
Abstract
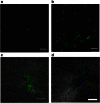
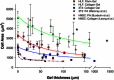
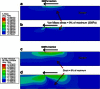
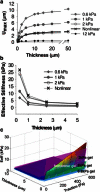
Recent observations suggest that cells on fibrous extracellular matrix materials sense mechanical signals over much larger distances than they do on linearly elastic synthetic materials. In this work, we systematically investigate the distance fibroblasts can sense a rigid boundary through fibrous gels by quantifying the spread areas of human lung fibroblasts and 3T3 fibroblasts cultured on sloped collagen and fibrin gels. The cell areas gradually decrease as gel thickness increases from 0 to 150 μm, with characteristic sensing distances of >65 μm below fibrin and collagen gels, and spreading affected on gels as thick as 150 μm. These results demonstrate that fibroblasts sense deeper into collagen and fibrin gels than they do into polyacrylamide gels, with the latter exhibiting characteristic sensing distances of <5 μm. We apply finite-element analysis to explore the role of strain stiffening, a characteristic mechanical property of collagen and fibrin that is not observed in polyacrylamide, in facilitating mechanosensing over long distances. Our analysis shows that the effective stiffness of both linear and nonlinear materials sharply increases once the thickness is reduced below 5 μm, with only a slight enhancement in sensitivity to depth for the nonlinear material at very low thickness and high applied traction. Multiscale simulations with a simplified geometry predict changes in fiber alignment deep into the gel and a large increase in effective stiffness with a decrease in substrate thickness that is not predicted by nonlinear elasticity. These results suggest that the observed cell-spreading response to gel thickness is not explained by the nonlinear strain-stiffening behavior of the material alone and is likely due to the fibrous nature of the proteins.
Copyright © 2013 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Yip C.Y., Chen J.H., Simmons C.A. Calcification by valve interstitial cells is regulated by the stiffness of the extracellular matrix. Arterioscler. Thromb. Vasc. Biol. 2009;29:936–942. - PubMed
-
- Wang H.B., Dembo M., Wang Y.L. Substrate flexibility regulates growth and apoptosis of normal but not transformed cells. Am. J. Physiol. Cell Physiol. 2000;279:C1345–C1350. - PubMed
-
- Ingber D.E. Mechanobiology and diseases of mechanotransduction. Ann. Med. 2003;35:564–577. - PubMed
-
- Vogel V., Sheetz M. Local force and geometry sensing regulate cell functions. Nat. Rev. Mol. Cell Biol. 2006;7:265–275. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

