Apical oscillations in amnioserosa cells: basolateral coupling and mechanical autonomy
- PMID: 23823245
- PMCID: PMC3699749
- DOI: 10.1016/j.bpj.2013.05.027
Apical oscillations in amnioserosa cells: basolateral coupling and mechanical autonomy
Abstract
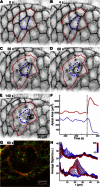
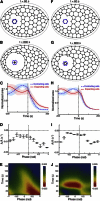
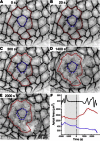
Holographic laser microsurgery is used to isolate single amnioserosa cells in vivo during early dorsal closure. During this stage of Drosophila embryogenesis, amnioserosa cells undergo oscillations in apical surface area. The postisolation behavior of individual cells depends on their preisolation phase in these contraction/expansion cycles: cells that were contracting tend to collapse quickly after isolation; cells that were expanding do not immediately collapse, but instead pause or even continue to expand for ∼40 s. In either case, the postisolation apical collapse can be prevented by prior anesthetization of the embryos with CO2. These results suggest that although the amnioserosa is under tension, its cells are subjected to only small elastic strains. Furthermore, their postisolation apical collapse is not a passive elastic relaxation, and both the contraction and expansion phases of their oscillations are driven by intracellular forces. All of the above require significant changes to existing computational models.
Copyright © 2013 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Fristrom D. The cellular basis of epithelial morphogenesis. A review. Tissue Cell. 1988;20:645–690. - PubMed
-
- Bard J. Cambridge University Press; Cambridge, UK: 1992. Morphogenesis: The Cellular and Molecular Processes of Developmental Anatomy. - PubMed
-
- Campos-Ortega J., Hartenstein V. 2nd Ed. Springer; New York: 1997. The Embryonic Development of Drosophila melanogaster.
-
- Lecuit T., Lenne P.-F. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nat. Rev. Mol. Cell Biol. 2007;8:633–644. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

