Mitigation of Lethal Radiation Syndrome in Mice by Intramuscular Injection of 3D Cultured Adherent Human Placental Stromal Cells
- PMID: 23823334
- PMCID: PMC3688917
- DOI: 10.1371/journal.pone.0066549
Mitigation of Lethal Radiation Syndrome in Mice by Intramuscular Injection of 3D Cultured Adherent Human Placental Stromal Cells
Abstract
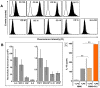
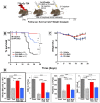
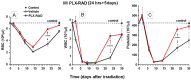
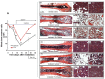
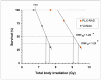
Exposure to high lethal dose of ionizing radiation results in acute radiation syndrome with deleterious systemic effects to different organs. A primary target is the highly sensitive bone marrow and the hematopoietic system. In the current study C3H/HeN mice were total body irradiated by 7.7 Gy. Twenty four hrs and 5 days after irradiation 2×10(6) cells from different preparations of human derived 3D expanded adherent placental stromal cells (PLX) were injected intramuscularly. Treatment with batches consisting of pure maternal cell preparations (PLX-Mat) increased the survival of the irradiated mice from ∼27% to 68% (P<0.001), while cell preparations with a mixture of maternal and fetal derived cells (PLX-RAD) increased the survival to ∼98% (P<0.0001). The dose modifying factor of this treatment for both 50% and 37% survival (DMF50 and DMF37) was∼1.23. Initiation of the more effective treatment with PLX-RAD injection could be delayed for up to 48 hrs after irradiation with similar effect. A delayed treatment by 72 hrs had lower, but still significantly effect (p<0.05). A faster recovery of the BM and improved reconstitution of all blood cell lineages in the PLX-RAD treated mice during the follow-up explains the increased survival of the cells treated irradiated mice. The number of CD45+/SCA1+ hematopoietic progenitor cells within the fast recovering population of nucleated BM cells in the irradiated mice was also elevated in the PLX-RAD treated mice. Our study suggests that IM treatment with PLX-RAD cells may serve as a highly effective "off the shelf" therapy to treat BM failure following total body exposure to high doses of radiation. The results suggest that similar treatments may be beneficial also for clinical conditions associated with severe BM aplasia and pancytopenia.
Conflict of interest statement
Figures



References
-
- Koenig KL, Goans RE, Hatchett RJ, Mettler FA Jr, Schumacher TA, et al. (2005) Medical treatment of radiological casualties: current concepts. Ann Emerg Med 45: 643–852. - PubMed
-
- Meineke V, Fliedner TM (2005) Radiation-induced multi-organ involvement and failure: challenges for radiation accident medical management and future research. BJR Suppl 27: 196–200.
-
- Need JT, Mothershead JL (2006) Strategic National Stockpile program: implications for military medicine. Mil Med 171: 698–702. - PubMed
-
- Andersson KG, Mikkelsen T, Astrup P, Thykier-Nielsen S, Jacobsen LH, et al. (2008) Estimation of health hazards resulting from a radiological terrorist attack in a city. Radiat Prot Dosimetry 131: 297–307. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

