Electrical stimulation as a biomimicry tool for regulating muscle cell behavior
- PMID: 23823664
- PMCID: PMC3812291
- DOI: 10.4161/org.25121
Electrical stimulation as a biomimicry tool for regulating muscle cell behavior
Abstract
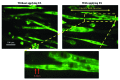
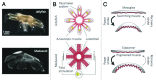
There is a growing need to understand muscle cell behaviors and to engineer muscle tissues to replace defective tissues in the body. Despite a long history of the clinical use of electric fields for muscle tissues in vivo, electrical stimulation (ES) has recently gained significant attention as a powerful tool for regulating muscle cell behaviors in vitro. ES aims to mimic the electrical environment of electroactive muscle cells (e.g., cardiac or skeletal muscle cells) by helping to regulate cell-cell and cell-extracellular matrix (ECM) interactions. As a result, it can be used to enhance the alignment and differentiation of skeletal or cardiac muscle cells and to aid in engineering of functional muscle tissues. Additionally, ES can be used to control and monitor force generation and electrophysiological activity of muscle tissues for bio-actuation and drug-screening applications in a simple, high-throughput, and reproducible manner. In this review paper, we briefly describe the importance of ES in regulating muscle cell behaviors in vitro, as well as the major challenges and prospective potential associated with ES in the context of muscle tissue engineering.
Keywords: Electrical stimulation; alignment; bio-actuators; differentiation; drug-screening models; muscle cells; muscle tissue engineering.
Figures
References
-
- Baar K, Birla R, Boluyt MO, Borschel GH, Arruda EM, Dennis RG. Self-organization of rat cardiac cells into contractile 3-D cardiac tissue. FASEB J. 2005;19:275–7. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
