The Expression of the fim Operon Is Crucial for the Survival of Streptococcus parasanguinis FW213 within Macrophages but Not Acid Tolerance
- PMID: 23823757
- PMCID: PMC3688865
- DOI: 10.1371/journal.pone.0066163
The Expression of the fim Operon Is Crucial for the Survival of Streptococcus parasanguinis FW213 within Macrophages but Not Acid Tolerance
Abstract
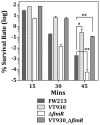
The acquisition of transition metal ions is essential for the viability and in some cases the expression of virulence genes in bacteria. The fimCBA operon of Streptococcus parasanguinis FW213 encodes a Mn(2+)/Fe(2+)-specific ATP-binding cassette transporter. FimA, a lipoprotein in the system, is essential for the development of endocarditis, presumably by binding to fibrin monolayers on the damaged heart tissue. Recent sequence analysis revealed that Spaf_0344 was homologous to Streptococcus gordonii scaR, encoding a metalloregulatory protein for the Sca Mn(2+)-specific transporter. Based on the homology, Spaf_0344 was designated fimR. By using various fim promoter (p fim ) derivatives fused with a promoterless chloramphenicol acetyltransferase gene, the functions of the cis-elements of p fim were analyzed in the wild-type and fimR-deficient hosts. The result indicated that FimR represses the expression of p fim and the palindromic sequences 5' to fimC are involved in repression of p fim . A direct interaction between FimR and the palindromic sequences was further confirmed by in vitro electrophoresis gel mobility shift assay and in vivo chromatin immunoprecipitation assay (ChIP)-quantitative real-time PCR (qPCR). The result of the ChIP-qPCR analysis also indicated that FimR is activated by Mn(2+) and, to a lesser degree, Fe(2+). Functional analysis indicated that the expression of FimA in S. parasanguinis was critical for wild-type levels of survival against oxidative stress and within phagocytes, but not for acid tolerance. Taken together, in addition to acting as an adhesin (FimA), the expression of the fim operon is critical for the pathogenic capacity of S. parasanguinis.
Conflict of interest statement
Figures





Similar articles
-
Functional Analysis of a Fibronectin Binding Protein of Streptococcus parasanguinis FW213.Curr Microbiol. 2020 Nov;77(11):3430-3440. doi: 10.1007/s00284-020-02152-7. Epub 2020 Aug 6. Curr Microbiol. 2020. PMID: 32761388
-
The divergently transcribed Streptococcus parasanguis virulence-associated fimA operon encoding an Mn(2+)-responsive metal transporter and pepO encoding a zinc metallopeptidase are not coordinately regulated.Infect Immun. 2002 Oct;70(10):5706-14. doi: 10.1128/IAI.70.10.5706-5714.2002. Infect Immun. 2002. PMID: 12228300 Free PMC article.
-
Functional Analysis of the Collagen Binding Proteins of Streptococcus parasanguinis FW213.mSphere. 2020 Oct 14;5(5):e00863-20. doi: 10.1128/mSphere.00863-20. mSphere. 2020. PMID: 33055259 Free PMC article.
-
Expression of the virulence-related Sca (Mn2+) permease in Streptococcus gordonii is regulated by a diphtheria toxin metallorepressor-like protein ScaR.Mol Microbiol. 2000 Oct;38(1):140-53. doi: 10.1046/j.1365-2958.2000.02122.x. Mol Microbiol. 2000. PMID: 11029696
-
The fimA locus of Streptococcus parasanguis encodes an ATP-binding membrane transport system.Mol Microbiol. 1995 Mar;15(5):849-63. doi: 10.1111/j.1365-2958.1995.tb02355.x. Mol Microbiol. 1995. PMID: 7596287
Cited by
-
The GlnR Regulon in Streptococcus mutans Is Differentially Regulated by GlnR and PmrA.PLoS One. 2016 Jul 25;11(7):e0159599. doi: 10.1371/journal.pone.0159599. eCollection 2016. PLoS One. 2016. PMID: 27454482 Free PMC article.
-
Macrophage Polarization Alters Postphagocytosis Survivability of the Commensal Streptococcus gordonii.Infect Immun. 2018 Feb 20;86(3):e00858-17. doi: 10.1128/IAI.00858-17. Print 2018 Mar. Infect Immun. 2018. PMID: 29229734 Free PMC article.
-
TroR Negatively Regulates the TroABCD System and Is Required for Resistance to Metal Toxicity and Virulence in Streptococcus suis.Appl Environ Microbiol. 2021 Sep 28;87(20):e0137521. doi: 10.1128/AEM.01375-21. Epub 2021 Aug 11. Appl Environ Microbiol. 2021. PMID: 34378993 Free PMC article.
-
Functional Analysis of a Fibronectin Binding Protein of Streptococcus parasanguinis FW213.Curr Microbiol. 2020 Nov;77(11):3430-3440. doi: 10.1007/s00284-020-02152-7. Epub 2020 Aug 6. Curr Microbiol. 2020. PMID: 32761388
-
Manganese transport by Streptococcus sanguinis in acidic conditions and its impact on growth in vitro and in vivo.Mol Microbiol. 2022 Feb;117(2):375-393. doi: 10.1111/mmi.14854. Epub 2021 Dec 18. Mol Microbiol. 2022. PMID: 34862691 Free PMC article.
References
-
- Carlsson J, Grahnen H, Jonsson G, Wikner S (1970) Establishment of Streptococcus sanguis in the mouths of infants. Arch Oral Biol 15: 1143–1148. - PubMed
-
- Jenkinson HF, Lamont RJ (1997) Streptococcal adhesion and colonization. Crit Rev Oral Biol Med 8: 175–200. - PubMed
-
- van der Meer JT, van Vianen W, Hu E, van Leeuwen WB, Valkenburg HA, et al. (1991) Distribution, antibiotic susceptibility and tolerance of bacterial isolates in culture-positive cases of endocarditis in The Netherlands. Eur J Clin Microbiol Infect Dis 10: 728–734. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

