Inhibition of the Fe(III)-catalyzed dopamine oxidation by ATP and its relevance to oxidative stress in Parkinson's disease
- PMID: 23823941
- PMCID: PMC3778429
- DOI: 10.1021/cn400105d
Inhibition of the Fe(III)-catalyzed dopamine oxidation by ATP and its relevance to oxidative stress in Parkinson's disease
Abstract
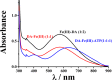
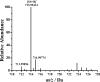
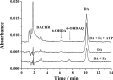
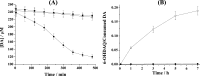
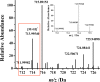
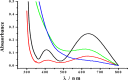
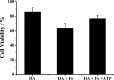
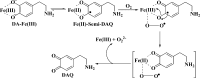
Parkinson's disease (PD) is characterized by the progressive degeneration of dopaminergic cells, which implicates a role of dopamine (DA) in the etiology of PD. A possible DA degradation pathway is the Fe(III)-catalyzed oxidation of DA by oxygen, which produces neuronal toxins as side products. We investigated how ATP, an abundant and ubiquitous molecule in cellular milieu, affects the catalytic oxidation reaction of dopamine. For the first time, a unique, highly stable DA-Fe(III)-ATP ternary complex was formed and characterized in vitro. ATP as a ligand shifts the catecholate-Fe(III) ligand metal charge transfer (LMCT) band to a longer wavelength and the redox potentials of both DA and the Fe(III) center in the ternary complex. Remarkably, the additional ligation by ATP was found to significantly reverse the catalytic effect of the Fe(III) center on the DA oxidation. The reversal is attributed to the full occupation of the Fe(III) coordination sites by ATP and DA, which blocks O2 from accessing the Fe(III) center and its further reaction with DA. The biological relevance of this complex is strongly implicated by the identification of the ternary complex in the substantia nigra of rat brain and its attenuation of cytotoxicity of the Fe(III)-DA complex. Since ATP deficiency accompanies PD and neurotoxin 1-methyl-4-phenylpyridinium (MPP(+)) induced PD, deficiency of ATP and the resultant impairment toward the inhibition of the Fe(III)-catalyzed DA oxidation may contribute to the pathogenesis of PD. Our finding provides new insight into the pathways of DA oxidation and its relationship with synaptic activity.
Figures


Similar articles
-
Norepinephrine-Fe(III)-ATP Ternary Complex and Its Relevance to Parkinson's Disease.ACS Chem Neurosci. 2019 Jun 19;10(6):2777-2785. doi: 10.1021/acschemneuro.9b00009. Epub 2019 May 13. ACS Chem Neurosci. 2019. PMID: 31059226
-
Elucidation of the interplay between Fe(II), Fe(III), and dopamine with relevance to iron solubilization and reactive oxygen species generation by catecholamines.J Neurochem. 2016 Jun;137(6):955-68. doi: 10.1111/jnc.13615. Epub 2016 May 12. J Neurochem. 2016. PMID: 26991725 Free PMC article.
-
The role of transition metals in the pathogenesis of Parkinson's disease.J Neurol Sci. 1995 Dec;134 Suppl:69-78. doi: 10.1016/0022-510x(95)00210-s. J Neurol Sci. 1995. PMID: 8847547
-
Converging roles of ion channels, calcium, metabolic stress, and activity pattern of Substantia nigra dopaminergic neurons in health and Parkinson's disease.J Neurochem. 2016 Oct;139 Suppl 1(Suppl Suppl 1):156-178. doi: 10.1111/jnc.13572. Epub 2016 Mar 23. J Neurochem. 2016. PMID: 26865375 Free PMC article. Review.
-
Interaction of alpha-synuclein and dopamine metabolites in the pathogenesis of Parkinson's disease: a case for the selective vulnerability of the substantia nigra.Acta Neuropathol. 2006 Aug;112(2):115-26. doi: 10.1007/s00401-006-0096-2. Epub 2006 Jun 22. Acta Neuropathol. 2006. PMID: 16791599 Review.
Cited by
-
Structure-redox-relaxivity relationships for redox responsive manganese-based magnetic resonance imaging probes.Inorg Chem. 2014 Oct 6;53(19):10748-61. doi: 10.1021/ic502005u. Epub 2014 Sep 16. Inorg Chem. 2014. PMID: 25226090 Free PMC article.
-
Kinetic Modeling of pH-Dependent Oxidation of Dopamine by Iron and Its Relevance to Parkinson's Disease.Front Neurosci. 2018 Nov 26;12:859. doi: 10.3389/fnins.2018.00859. eCollection 2018. Front Neurosci. 2018. PMID: 30534046 Free PMC article.
-
Mitochondrial Dynamics Impairment in Dexamethasone-Treated Neuronal Cells.Neurochem Res. 2019 Jul;44(7):1567-1581. doi: 10.1007/s11064-019-02779-4. Epub 2019 Mar 19. Neurochem Res. 2019. PMID: 30888577
-
Astrocytic Oxidative/Nitrosative Stress Contributes to Parkinson's Disease Pathogenesis: The Dual Role of Reactive Astrocytes.Antioxidants (Basel). 2019 Aug 1;8(8):265. doi: 10.3390/antiox8080265. Antioxidants (Basel). 2019. PMID: 31374936 Free PMC article. Review.
-
Identification of distinct blood-based biomarkers in early stage of Parkinson's disease.Neurol Sci. 2020 Apr;41(4):893-901. doi: 10.1007/s10072-019-04165-y. Epub 2019 Dec 11. Neurol Sci. 2020. PMID: 31828678
References
-
- Moore D. J.; West A. B.; Dawson V. L.; Dawson T. M. (2005) Molecular pathophysiology of Parkinson′s disease. Annu. Rev. Neurosci. 28, 57–87. - PubMed
-
- Spillantini M. G.; Schmidt M. L.; Lee V. M.; Trojanowski J. Q.; Jakes R.; Goedert M. (1997) a-Synuclein in Lewy bodies. Nature 388, 839–840. - PubMed
-
- Luo Y.; Umegaki H.; Wang X.; Abe R.; Roth G. S. (1998) Dopamine induces apoptosis through an oxidation-involved SAPK/JNK activation pathway. J. Biol. Chem. 273, 3756–3764. - PubMed
-
- Irwin I., and Langston J. W. (1995) Endogenous toxins as potential etiologic agents in Parkinson′s disease, pp 153–201, Marcel Dekker, New York.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

