Prediction of non-alcoholic fatty-liver disease and liver fat content by serum molecular lipids
- PMID: 23824212
- PMCID: PMC3764317
- DOI: 10.1007/s00125-013-2981-2
Prediction of non-alcoholic fatty-liver disease and liver fat content by serum molecular lipids
Abstract
Aims/hypothesis: We examined whether analysis of lipids by ultra-performance liquid chromatography (UPLC) coupled to MS allows the development of a laboratory test for non-alcoholic fatty-liver disease (NAFLD), and how a lipid-profile biomarker compares with the prediction of NAFLD and liver-fat content based on routinely available clinical and laboratory data.
Methods: We analysed the concentrations of molecular lipids by UPLC-MS in blood samples of 679 well-characterised individuals in whom liver-fat content was measured using proton magnetic resonance spectroscopy ((1)H-MRS) or liver biopsy. The participants were divided into biomarker-discovery (n = 287) and validation (n = 392) groups to build and validate the diagnostic models, respectively.
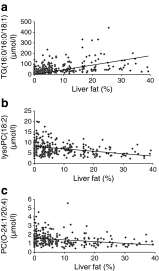
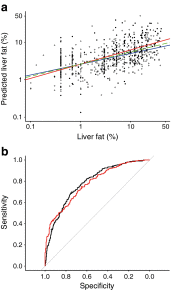
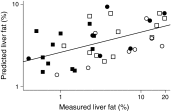
Results: Individuals with NAFLD had increased triacylglycerols with low carbon number and double-bond content while lysophosphatidylcholines and ether phospholipids were diminished in those with NAFLD. A serum-lipid signature comprising three molecular lipids ('lipid triplet') was developed to estimate the percentage of liver fat. It had a sensitivity of 69.1% and specificity of 73.8% when applied for diagnosis of NAFLD in the validation series. The usefulness of the lipid triplet was demonstrated in a weight-loss intervention study.
Conclusions/interpretation: The liver-fat-biomarker signature based on molecular lipids may provide a non-invasive tool to diagnose NAFLD, in addition to highlighting lipid molecular pathways involved in the disease.
Figures
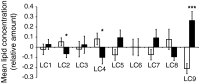
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

