Traditional and new influenza vaccines
- PMID: 23824369
- PMCID: PMC3719499
- DOI: 10.1128/CMR.00097-12
Traditional and new influenza vaccines
Abstract
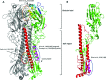
The challenges in successful vaccination against influenza using conventional approaches lie in their variable efficacy in different age populations, the antigenic variability of the circulating virus, and the production and manufacturing limitations to ensure safe, timely, and adequate supply of vaccine. The conventional influenza vaccine platform is based on stimulating immunity against the major neutralizing antibody target, hemagglutinin (HA), by virus attenuation or inactivation. Improvements to this conventional system have focused primarily on improving production and immunogenicity. Cell culture, reverse genetics, and baculovirus expression technology allow for safe and scalable production, while adjuvants, dose variation, and alternate routes of delivery aim to improve vaccine immunogenicity. Fundamentally different approaches that are currently under development hope to signal new generations of influenza vaccines. Such approaches target nonvariable regions of antigenic proteins, with the idea of stimulating cross-protective antibodies and thus creating a "universal" influenza vaccine. While such approaches have obvious benefits, there are many hurdles yet to clear. Here, we discuss the process and challenges of the current influenza vaccine platform as well as new approaches that are being investigated based on the same antigenic target and newer technologies based on different antigenic targets.
Figures



References
-
- Centers for Disease Control and Prevention 2010. Estimates of deaths associated with seasonal influenza—United States, 1976-2007. MMWR Morb. Mortal. Wkly. Rep. 59:1057–1062 - PubMed
-
- Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, Fukuda K. 2004. Influenza-associated hospitalizations in the United States. JAMA 292:1333–1340 - PubMed
-
- Nair H, Brooks WA, Katz M, Roca A, Berkley JA, Madhi SA, Simmerman JM, Gordon A, Sato M, Howie S, Krishnan A, Ope M, Lindblade KA, Carosone-Link P, Lucero M, Ochieng W, Kamimoto L, Dueger E, Bhat N, Vong S, Theodoratou E, Chittaganpitch M, Chimah O, Balmaseda A, Buchy P, Harris E, Evans V, Katayose M, Gaur B, O'Callaghan-Gordo C, Goswami D, Arvelo W, Venter M, Briese T, Tokarz R, Widdowson MA, Mounts AW, Breiman RF, Feikin DR, Klugman KP, Olsen SJ, Gessner BD, Wright PF, Rudan I, Broor S, Simoes EA, Campbell H. 2011. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet 378:1917–1930 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

