Pasteurella multocida: from zoonosis to cellular microbiology
- PMID: 23824375
- PMCID: PMC3719492
- DOI: 10.1128/CMR.00024-13
Pasteurella multocida: from zoonosis to cellular microbiology
Abstract
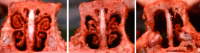
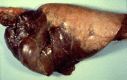
In a world where most emerging and reemerging infectious diseases are zoonotic in nature and our contacts with both domestic and wild animals abound, there is growing awareness of the potential for human acquisition of animal diseases. Like other Pasteurellaceae, Pasteurella species are highly prevalent among animal populations, where they are often found as part of the normal microbiota of the oral, nasopharyngeal, and upper respiratory tracts. Many Pasteurella species are opportunistic pathogens that can cause endemic disease and are associated increasingly with epizootic outbreaks. Zoonotic transmission to humans usually occurs through animal bites or contact with nasal secretions, with P. multocida being the most prevalent isolate observed in human infections. Here we review recent comparative genomics and molecular pathogenesis studies that have advanced our understanding of the multiple virulence mechanisms employed by Pasteurella species to establish acute and chronic infections. We also summarize efforts being explored to enhance our ability to rapidly and accurately identify and distinguish among clinical isolates and to control pasteurellosis by improved development of new vaccines and treatment regimens.
Figures


References
-
- Kuiken T, Leighton FA, Fouchier RA, LeDuc JW, Peiris JS, Schudel A, Stohr K, Osterhaus AD. 2005. Public health. Pathogen surveillance in animals. Science 309:1680–1681 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

