Dissecting phenotypic traits linked to human resilience to Alzheimer's pathology
- PMID: 23824488
- PMCID: PMC3722351
- DOI: 10.1093/brain/awt171
Dissecting phenotypic traits linked to human resilience to Alzheimer's pathology
Abstract
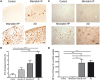
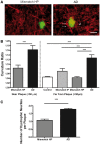
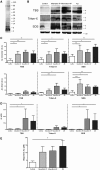
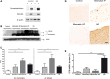
Clinico-pathological correlation studies and positron emission tomography amyloid imaging studies have shown that some individuals can tolerate substantial amounts of Alzheimer's pathology in their brains without experiencing dementia. Few details are known about the neuropathological phenotype of these unique cases that might prove relevant to understanding human resilience to Alzheimer's pathology. We conducted detailed quantitative histopathological and biochemical assessments on brains from non-demented individuals before death whose brains were free of substantial Alzheimer's pathology, non-demented individuals before death but whose post-mortem examination demonstrated significant amounts of Alzheimer's changes ('mismatches'), and demented Alzheimer's cases. Quantification of amyloid-β plaque burden, stereologically-based counts of neurofibrillary tangles, neurons and reactive glia, and morphological analyses of axons were performed in the multimodal association cortex lining the superior temporal sulcus. Levels of synaptic integrity markers, and soluble monomeric and multimeric amyloid-β and tau species were measured. Our results indicate that some individuals can accumulate equivalent loads of amyloid-β plaques and tangles to those found in demented Alzheimer's cases without experiencing dementia. Analyses revealed four main phenotypic differences among these two groups: (i) mismatches had striking preservation of neuron numbers, synaptic markers and axonal geometry compared to demented cases; (ii) demented cases had significantly higher burdens of fibrillar thioflavin-S-positive plaques and of oligomeric amyloid-β deposits reactive to conformer-specific antibody NAB61 than mismatches; (iii) strong and selective accumulation of hyperphosphorylated soluble tau multimers into the synaptic compartment was noted in demented cases compared with controls but not in mismatches; and (iv) the robust glial activation accompanying amyloid-β and tau pathologies in demented cases was remarkably reduced in mismatches. Further biochemical measurements of soluble amyloid-β species-monomers, dimers and higher molecular weight oligomers-in total brain homogenates and synaptoneurosomal preparations failed to demonstrate significant differences between mismatches and demented cases. Together, these data suggest that amyloid-β plaques and tangles do not inevitably result in neural system derangement and dementia in all individuals. We identified distinct phenotypic characteristics in the profile of brain fibrillar and soluble amyloid-β and tau accrual and in the glial response that discriminated demented and non-demented individuals with high loads of Alzheimer's pathology. Amyloid-β deposition in the form of fibrillar plaques and intimately related oligomeric amyloid-β assemblies, hyperphosphorylated soluble tau species localized in synapses, and glial activation emerged in this series as likely mediators of neurotoxicity and altered cognition, providing further insight into factors and pathways potentially involved in human susceptibility or resilience to Alzheimer's pathological changes.
Keywords: Alzheimers disease; amyloid pathology; astrocytes; microglia; resilience; tau pathology.
Figures



References
-
- Arias E. United States life tables, 2008. Natl Vital Stat Rep. 2012;61:3. - PubMed
-
- Arriagada PV, Marzloff K, Hyman BT. Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer's disease. Neurology. 1992;42:1681–88. - PubMed
-
- Benilova I, Karran E, De SB. The toxic Abeta oligomer and Alzheimer's disease: an emperor in need of clothes. Nat Neurosci. 2012;15:349–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

