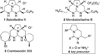
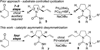
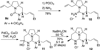
Desymmetrization of meso-2,5-diallylpyrrolidinyl ureas through asymmetric palladium-catalyzed carboamination: stereocontrolled synthesis of bicyclic ureas
- PMID: 23824590
- PMCID: PMC3812936
- DOI: 10.1002/anie.201302720
Desymmetrization of meso-2,5-diallylpyrrolidinyl ureas through asymmetric palladium-catalyzed carboamination: stereocontrolled synthesis of bicyclic ureas
Abstract
The stereoselective synthesis of fused bicyclic ureas 8 is accomplished via enantioselective Pd-catalyzed desymmetrizing carboamination reactions of meso-2,5-diallylpyrroldinyl urea 7c. The reactions generate a C–N bond, a C–C bond, and afford products bearing three stereocenters with good diastereoselectivity (6–12:1 dr) and enantioselectivity (up to 95:5 er). The N-(p-chlorophenyl) group can be cleaved in good yield using a two step sequence. In addition, 8c was transformed to a tricyclic guanidine product using a four-step (two pot) procedure and was converted to 9-epi-batzelladine k in seven steps.
Keywords: alkaloids; asymmetric catalysis; desymmetrization; heterocycles; palladium.
Figures
References
-
-
For reviews, see: Enriquez-Garcia A, Kündig EP. Chem. Soc. Rev. 2012;41:7803–7831. Diaz de Villegas MD, Galvez JA, Etayo P, Badorrey R, Lopez Ram de Viu P. Chem. Soc. Rev. 2011;40:5564–5587. Rovis T. In: New Frontiers in Asymmetric Catalysis. Mikami K, Lautens M, editors. Hoboken, NJ: Wiley; 2007. pp. 275–309. Mikami K, Yoshida A. J. Synth. Org. Chem., Jpn. 2002;60:732–739. Trost BM. Isr. J. Chem. 1997;37:109–118.
-
-
- Berlinck RGS, Trindade-Silva AE, Santos MFC. Nat. Prod. Rep. 2012;29:1382–1406. - PubMed
- Berlinck RGS, Burtoloso ACB, Trindade-Silva AE, Romminger S, Morais RP, Bandeira K, Mizuno CM. Nat. Prod. Rep. 2010;27:1871–1907. - PubMed
- Berlinck RGS, Burtoloso ACB, Kossuga MH. Nat. Prod. Rep. 2008;25:919–954. - PubMed
- Berlinck RGS, Kossuga MH. Nat. Prod. Rep. 2005;22:516–550. - PubMed
- Bewley CA, Ray S, Cohen F, Collins SK, Overman LE. J. Nat. Prod. 2004;67:1319–1324. - PubMed
-
- Laville R, Thomas OP, Berrue F, Marquez D, Vacelet J, Amade P. J. Nat. Prod. 2009;72:1589–1594. - PubMed
- Gallimore WA, Kelly M, Scheuer PJ. J. Nat. Prod. 2005;68:1420–1423. - PubMed
- Hua H–M, Peng J, Dunbar DC, Schinazi RF, de Castro Andrews AG, Cuevas C, Garcia-Fernandez LF, Kelly M, Hamann MT. Tetrahedron. 2007;63:11179–11188.
- Patil AD, Kumar NV, Kokke WC, Bean MF, Freyer AJ, De Brosse C, Mai S, Truneh A, Faulkner DJ, Carte B, Breen AL, Hertzberg RP, Johnson RK, Westley JW, Potts BCM. J. Org. Chem. 1995;60:1182–1188.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources