Galectin-4 Reduces Migration and Metastasis Formation of Pancreatic Cancer Cells
- PMID: 23824659
- PMCID: PMC3688853
- DOI: 10.1371/journal.pone.0065957
Galectin-4 Reduces Migration and Metastasis Formation of Pancreatic Cancer Cells
Abstract
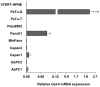
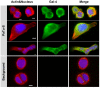
Galectin-4 (Gal-4) is a member of the galectin family of glycan binding proteins that shows a significantly higher expression in cystic tumors of the human pancreas and in pancreatic adenocarcinomas compared to normal pancreas. However, the putative function of Gal-4 in tumor progression of pancreatic cancer is still incompletely understood. In this study the role of Gal-4 in cancer progression was investigated, using a set of defined pancreatic cancer cell lines, Pa-Tu-8988S (PaTu-S) and Pa-Tu-8988T (PaTu-T), as a model. These two cell lines are derived from the same liver metastasis of a human primary pancreatic adenocarcinoma, but differ in their growth characteristics and metastatic capacity. We demonstrated that Gal-4 expression is high in PaTu-S, which shows poor migratory properties, whereas much lower Gal-4 levels are observed in the highly metastatic cell line PaTu-T. In PaTu-S, Gal-4 is found in the cytoplasm, but it is also secreted and accumulates at the membrane at sites of contact with neighboring cells. Moreover, we show that Gal-4 inhibits metastasis formation by delaying migration of pancreatic cancer cells in vitro using a scratch assay, and in vivo using zebrafish (Danio rerio) as an experimental model. Our data suggest that Gal-4 may act at the cell-surface of PaTu-S as an adhesion molecule to prevent release of the tumor cells, but has in addition a cytosolic function by inhibiting migration via a yet unknown mechanism.
Conflict of interest statement
Figures




References
-
- Krejs GJ (2010) Pancreatic cancer: epidemiology and risk factors. Dig Dis 28: 355–358. - PubMed
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, et al. (2009) Cancer statistics, 2009. CA Cancer J Clin 59: 225–249. - PubMed
-
- Danguy A, Camby I, Kiss R (2002) Galectins and cancer. Biochim Biophys Acta 1572: 285–293. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

