Src Dependent Pancreatic Acinar Injury Can Be Initiated Independent of an Increase in Cytosolic Calcium
- PMID: 23824669
- PMCID: PMC3688910
- DOI: 10.1371/journal.pone.0066471
Src Dependent Pancreatic Acinar Injury Can Be Initiated Independent of an Increase in Cytosolic Calcium
Abstract
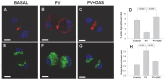
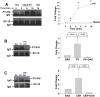
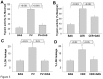
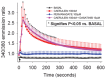
Several deleterious intra-acinar phenomena are simultaneously triggered on initiating acute pancreatitis. These culminate in acinar injury or inflammatory mediator generation in vitro and parenchymal damage in vivo. Supraphysiologic caerulein is one such initiator which simultaneously activates numerous signaling pathways including non-receptor tyrosine kinases such as of the Src family. It also causes a sustained increase in cytosolic calcium- a player thought to be crucial in regulating deleterious phenomena. We have shown Src to be involved in caerulein induced actin remodeling, and caerulein induced changes in the Golgi and post-Golgi trafficking to be involved in trypsinogen activation, which initiates acinar cell injury. However, it remains unclear whether an increase in cytosolic calcium is necessary to initiate acinar injury or if injury can be initiated at basal cytosolic calcium levels by an alternate pathway. To study the interplay between tyrosine kinase signaling and calcium, we treated mouse pancreatic acinar cells with the tyrosine phosphatase inhibitor pervanadate. We studied the effect of the clinically used Src inhibitor Dasatinib (BMS-354825) on pervanadate or caerulein induced changes in Src activation, trypsinogen activation, cell injury, upstream cytosolic calcium, actin and Golgi morphology. Pervanadate, like supraphysiologic caerulein, induced Src activation, redistribution of the F-actin from its normal location in the sub-apical area to the basolateral areas, and caused antegrade fragmentation of the Golgi. These changes, like those induced by supraphysiologic caerulein, were associated with trypsinogen activation and acinar injury, all of which were prevented by Dasatinib. Interestingly, however, pervanadate did not cause an increase in cytosolic calcium, and the caerulein induced increase in cytosolic calcium was not affected by Dasatinib. These findings suggest that intra-acinar deleterious phenomena may be initiated independent of an increase in cytosolic calcium. Other players resulting in acinar injury along with the Src family of tyrosine kinases remain to be explored.
Conflict of interest statement
Figures
Similar articles
-
Phosphorylated guanine nucleotide exchange factor C3G, induced by pervanadate and Src family kinases localizes to the Golgi and subcortical actin cytoskeleton.BMC Cell Biol. 2004 Aug 20;5:31. doi: 10.1186/1471-2121-5-31. BMC Cell Biol. 2004. PMID: 15320955 Free PMC article.
-
Hydrogen sulfide induces ICAM-1 expression and neutrophil adhesion to caerulein-treated pancreatic acinar cells through NF-kappaB and Src-family kinases pathway.Exp Cell Res. 2010 May 15;316(9):1625-36. doi: 10.1016/j.yexcr.2010.02.044. Epub 2010 Mar 6. Exp Cell Res. 2010. PMID: 20211170
-
Hypothermia slows sequential and parallel steps initiated during caerulein pancreatitis.Pancreatology. 2014 Nov-Dec;14(6):459-64. doi: 10.1016/j.pan.2014.06.006. Epub 2014 Jul 3. Pancreatology. 2014. PMID: 25459565
-
Pathogenic mechanisms of acute pancreatitis.Curr Opin Gastroenterol. 2012 Sep;28(5):507-15. doi: 10.1097/MOG.0b013e3283567f52. Curr Opin Gastroenterol. 2012. PMID: 22885948 Free PMC article. Review.
-
The role of calcium in acute pancreatitis.Surgery. 2012 Sep;152(3 Suppl 1):S157-63. doi: 10.1016/j.surg.2012.05.013. Surgery. 2012. PMID: 22906890 Review.
Cited by
-
c-Src regulates cargo transit via the Golgi in pancreatic acinar cells.Sci Rep. 2018 Aug 9;8(1):11903. doi: 10.1038/s41598-018-30370-4. Sci Rep. 2018. PMID: 30093675 Free PMC article.
-
Elucidation of the roles of the Src kinases in pancreatic acinar cell signaling.J Cell Biochem. 2015 Jan;116(1):22-36. doi: 10.1002/jcb.24895. J Cell Biochem. 2015. PMID: 25079913 Free PMC article.
-
Src kinases play a novel dual role in acute pancreatitis affecting severity but no role in stimulated enzyme secretion.Am J Physiol Gastrointest Liver Physiol. 2016 Jun 1;310(11):G1015-27. doi: 10.1152/ajpgi.00349.2015. Epub 2016 Mar 31. Am J Physiol Gastrointest Liver Physiol. 2016. PMID: 27033118 Free PMC article.
-
Fatty Acid Ethyl Esters Are Less Toxic Than Their Parent Fatty Acids Generated during Acute Pancreatitis.Am J Pathol. 2016 Apr;186(4):874-84. doi: 10.1016/j.ajpath.2015.11.022. Epub 2016 Feb 12. Am J Pathol. 2016. PMID: 26878214 Free PMC article.
-
Characterization and Predictive Value of Near Infrared 2-Deoxyglucose Optical Imaging in Severe Acute Pancreatitis.PLoS One. 2016 Feb 22;11(2):e0149073. doi: 10.1371/journal.pone.0149073. eCollection 2016. PLoS One. 2016. PMID: 26901564 Free PMC article.
References
-
- Pandol SJ, Saluja AK, Imrie CW, Banks PA (2007) Acute pancreatitis: bench to the bedside. Gastroenterology 132(3): 1127–1151. - PubMed
-
- Grady T, Liang P, Ernst SA, Logsdon CD (1997) Chemokine gene expression in rat pancreatic acinar cells is an early event associated with acute pancreatitis. Gastroenterology 113(6): 1966–1975. - PubMed
-
- Han B, Ji B, Logsdon CD (2001) CCK independently activates intracellular trypsinogen and NF-kappaB in rat pancreatic acinar cells. Am J Physiol Cell Physiol 280(3): C465–472. - PubMed
-
- Gukovskaya AS, Gukovsky I, Jung Y, Mouria M, Pandol SJ (2002) Cholecystokinin induces caspase activation and mitochondrial dysfunction in pancreatic acinar cells. Roles in cell injury processes of pancreatitis. J Biol Chem 277(25): 22595–22604. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

