Mutational analysis of the binding pockets of the diketo acid inhibitor L-742,001 in the influenza virus PA endonuclease
- PMID: 23824822
- PMCID: PMC3807387
- DOI: 10.1128/JVI.00832-13
Mutational analysis of the binding pockets of the diketo acid inhibitor L-742,001 in the influenza virus PA endonuclease
Abstract
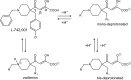
The influenza virus PA endonuclease, which cleaves capped host pre-mRNAs to initiate synthesis of viral mRNA, is a prime target for antiviral therapy. The diketo acid compound L-742,001 was previously identified as a potent inhibitor of the influenza virus endonuclease reaction, but information on its precise binding mode to PA or potential resistance profile is limited. Computer-assisted docking of L-742,001 into the crystal structure of inhibitor-free N-terminal PA (PA-Nter) indicated a binding orientation distinct from that seen in a recent crystallographic study with L-742,001-bound PA-Nter (R. M. DuBois et al., PLoS Pathog. 8:e1002830, 2012). A comprehensive mutational analysis was performed to determine which amino acid changes within the catalytic center of PA or its surrounding hydrophobic pockets alter the antiviral sensitivity to L-742,001 in cell culture. Marked (up to 20-fold) resistance to L-742,001 was observed for the H41A, I120T, and G81F/V/T mutant forms of PA. Two- to 3-fold resistance was seen for the T20A, L42T, and V122T mutants, and the R124Q and Y130A mutants were 3-fold more sensitive to L-742,001. Several mutations situated at noncatalytic sites in PA had no or only marginal impact on the enzymatic functionality of viral ribonucleoprotein complexes reconstituted in cell culture, consistent with the less conserved nature of these PA residues. Our data provide relevant insights into the binding mode of L-742,001 in the PA endonuclease active site. In addition, we predict some potential resistance sites that should be taken into account during optimization of PA endonuclease inhibitors toward tight binding in any of the hydrophobic pockets surrounding the catalytic center of the enzyme.
Figures



References
-
- Smith GJ, Vijaykrishna D, Bahl J, Lycett SJ, Worobey M, Pybus OG, Ma SK, Cheung CL, Raghwani J, Bhatt S, Peiris JS, Guan Y, Rambaut A. 2009. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature 459:1122–1125 - PubMed
-
- Simonsen L, Taylor RJ, Viboud C, Miller MA, Jackson LA. 2007. Mortality benefits of influenza vaccination in elderly people: an ongoing controversy. Lancet Infect. Dis. 7:658–666 - PubMed
-
- Deyde VM, Xu X, Bright RA, Shaw M, Smith CB, Zhang Y, Shu Y, Gubareva LV, Cox NJ, Klimov AI. 2007. Surveillance of resistance to adamantanes among influenza A(H3N2) and A(H1N1) viruses isolated worldwide. J. Infect. Dis. 196:249–257 - PubMed
-
- Moscona A. 2009. Global transmission of oseltamivir-resistant influenza. N. Engl. J. Med. 360:953–956 - PubMed
-
- Hayden F. 2009. Developing new antiviral agents for influenza treatment: what does the future hold? Clin. Infect. Dis. 48(Suppl 1):S3–S13 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

