Deficiency of ALADIN impairs redox homeostasis in human adrenal cells and inhibits steroidogenesis
- PMID: 23825130
- PMCID: PMC3958737
- DOI: 10.1210/en.2013-1241
Deficiency of ALADIN impairs redox homeostasis in human adrenal cells and inhibits steroidogenesis
Abstract
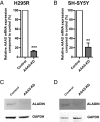
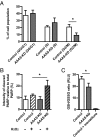
Triple A syndrome is a rare, autosomal recessive cause of adrenal failure. Additional features include alacrima, achalasia of the esophageal cardia, and progressive neurodegenerative disease. The AAAS gene product is the nuclear pore complex protein alacrima-achalasia-adrenal insufficiency neurological disorder (ALADIN), of unknown function. Triple A syndrome patient dermal fibroblasts appear to be more sensitive to oxidative stress than wild-type fibroblasts. To provide an adrenal and neuronal-specific disease model, we established AAAS-gene knockdown in H295R human adrenocortical tumor cells and SH-SY5Y human neuroblastoma cells by lentiviral short hairpin RNA transduction. AAAS-knockdown significantly reduced cell viability in H295R cells. This effect was exacerbated by hydrogen peroxide treatment and improved by application of the antioxidant N-acetylcysteine. An imbalance in redox homeostasis after AAAS knockdown was further suggested in the H295R cells by a decrease in the ratio of reduced to oxidized glutathione. AAAS-knockdown SH-SY5Y cells were also hypersensitive to oxidative stress and responded to antioxidant treatment. A further impact on function was observed in the AAAS-knockdown H295R cells with reduced expression of key components of the steroidogenic pathway, including steroidogenic acute regulatory and P450c11β protein expression. Importantly a significant reduction in cortisol production was demonstrated with AAAS knockdown, which was partially reversed with N-acetylcysteine treatment.
Conclusion: Our in vitro data in AAAS-knockdown adrenal and neuronal cells not only corroborates previous studies implicating oxidative stress in this disorder but also provides further insights into the pathogenic mechanisms in triple A syndrome.
Figures



References
-
- Allgrove J, Clayden GS, Grant DB, Macaulay JC. Familial glucocorticoid deficiency with achalasia of the cardia and deficient tear production. Lancet. 1978;1:1284–1286 - PubMed
-
- Handschug K, Sperling S, Yoon SJ, Hennig S, Clark AJ, Huebner A. Triple A syndrome is caused by mutations in AAAS, a new WD-repeat protein gene. Hum Mol Genet. 2001;10:283–290 - PubMed
-
- Gazarian M, Cowell CT, Bonney M, Grigor WG. The '4A' syndrome: adrenocortical insufficiency associated with achalasia, alacrima, autonomic and other neurological abnormalities. Eur J Pediatr. 1995;154:18–23 - PubMed
-
- Huebner A, Elias LL, Clark AJ. ACTH resistant syndromes. J Pediatr Endocrinol Metab. 1999;12:277–293 - PubMed
-
- Vallet A-E, Verschueren A, Petiot P, et al. Neurological features in adult Triple-A (Allgrove) syndrome. J Neurol. 2012;259:39–46 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

