Modification of clearview tuberculosis (TB) enzyme-linked immunosorbent assay for TB patients not infected with HIV
- PMID: 23825194
- PMCID: PMC3889579
- DOI: 10.1128/CVI.00375-13
Modification of clearview tuberculosis (TB) enzyme-linked immunosorbent assay for TB patients not infected with HIV
Abstract
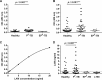
Diagnosis of active tuberculosis by detection of urinary lipoarabinomannan (uLAM) from Mycobacterium tuberculosis is an attractive approach. Concentrating urine 100-fold allowed quantitation of uLAM at levels equal to picograms/ml of nonconcentrated urine. The approach of concentrating urine 100-fold improved the clinical sensitivity of the Clearview TB enzyme-linked immunosorbent assay (ELISA) from 7% to 57% yet impaired its specificity from 97% to 89%. (This study has been registered at University Hospital of Turku under registration no. 47/180/2009, Helsinki University Central Hospital under 149/2010, University Hospital of Kuopio under 105/2010, and China Medical University Hospital, Taichung, under DMR-99-IRB-075-2.).
Figures
References
-
- Alere Inc 2013. BinaxNOW Legionella test manual. Alere Inc., Waltham, MA
-
- Alere Inc 2013. BinaxNOW S. pneumoniae test manual. Alere Inc., Waltham, MA
-
- Tam FC, Ling TK, Wong KT, Leung DT, Chan RC, Lim PL. 2008. The TUBEX test detects not only typhoid-specific antibodies but also soluble antigens and whole bacteria. J. Med. Microbiol. 57:316–323 - PubMed
-
- Minion J, Leung E, Talbot E, Dheda K, Pai M, Menzies D. 2011. Diagnosing tuberculosis with urine lipoarabinomannan: systematic review and meta-analysis. Eur. Respir. J. 38:1398–1405 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

