Muscle uncoupling protein 3 overexpression mimics endurance training and reduces circulating biomarkers of incomplete β-oxidation
- PMID: 23825224
- PMCID: PMC4046190
- DOI: 10.1096/fj.13-234302
Muscle uncoupling protein 3 overexpression mimics endurance training and reduces circulating biomarkers of incomplete β-oxidation
Erratum in
- FASEB J. 2014 Jan;28(1):520
Abstract
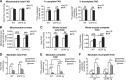
Exercise substantially improves metabolic health, making the elicited mechanisms important targets for novel therapeutic strategies. Uncoupling protein 3 (UCP3) is a mitochondrial inner membrane protein highly selectively expressed in skeletal muscle. Here we report that moderate UCP3 overexpression (roughly 3-fold) in muscles of UCP3 transgenic (UCP3 Tg) mice acts as an exercise mimetic in many ways. UCP3 overexpression increased spontaneous activity (∼40%) and energy expenditure (∼5-10%) and decreased oxidative stress (∼15-20%), similar to exercise training in wild-type (WT) mice. The increase in complete fatty acid oxidation (FAO; ∼30% for WT and ∼70% for UCP3 Tg) and energy expenditure (∼8% for WT and 15% for UCP3 Tg) in response to endurance training was higher in UCP3 Tg than in WT mice, showing an additive effect of UCP3 and endurance training on these two parameters. Moreover, increases in circulating short-chain acylcarnitines in response to acute exercise in untrained WT mice were absent with training or in UCP3 Tg mice. UCP3 overexpression had the same effect as training in decreasing long-chain acylcarnitines. Outcomes coincided with a reduction in muscle carnitine acetyltransferase activity that catalyzes the formation of acylcarnitines. Overall, results are consistent with the conclusions that circulating acylcarnitines could be used as a marker of incomplete muscle FAO and that UCP3 is a potential target for the treatment of prevalent metabolic diseases in which muscle FAO is affected.
Keywords: acylcarnitines; exercise mimetic; fatty acid oxidation; mitochondria; oxidative stress.
Conflict of interest statement
The authors thank Linda Jui for muscle fiber typing, Mahmoud Salkhordeh, Jian Xuan, Jaehoon Kim, Mine Palazoglu, Massud Atta, Kristie Cloos, and Wan Tan for their excellent technical assistance, Trina Knotts (Mouse Metabolomic Phenotyping Center, University of California, Davis, CA, USA) for ANCOVA analyses, and Dolors Serra (University of Barcelona, Barcelona, Spain) for her generous gift of anti-CrAT antibody.
Figures




References
-
- Pedersen B. K., Saltin B. (2006) Evidence for prescribing exercise as therapy in chronic disease. Scand. J. Med. Sci. Sports (Suppl. 1), 3–63 - PubMed
-
- Egan B., Zierath J. R.. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. , 162–184 - PubMed
-
- Calvo J. A., Daniels T. G., Wang X., Paul A., Lin J., Spiegelman B. M., Stevenson S. C., Rangwala S. M. (2008) Muscle-specific expression of PPARγ coactivator-1α improves exercise performance and increases peak oxygen uptake. J. Appl. Physiol. , 1304–1312 - PubMed
-
- Bostrom P., Wu J., Jedrychowski M. P., Korde A., Ye L., Lo J. C., Rasbach K. A., Bostrom E. A., Choi J. H., Long J. Z., Kajimura S., Zingaretti M. C., Vind B. F., Tu H., Cinti S., Hojlund K., Gygi S. P., Spiegelman B. M. (2012) A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature , 463–468 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

