Bactericidal antibiotics induce mitochondrial dysfunction and oxidative damage in Mammalian cells
- PMID: 23825301
- PMCID: PMC3760005
- DOI: 10.1126/scitranslmed.3006055
Bactericidal antibiotics induce mitochondrial dysfunction and oxidative damage in Mammalian cells
Abstract
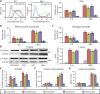
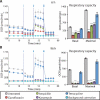
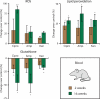
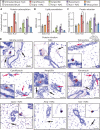
Prolonged antibiotic treatment can lead to detrimental side effects in patients, including ototoxicity, nephrotoxicity, and tendinopathy, yet the mechanisms underlying the effects of antibiotics in mammalian systems remain unclear. It has been suggested that bactericidal antibiotics induce the formation of toxic reactive oxygen species (ROS) in bacteria. We show that clinically relevant doses of bactericidal antibiotics-quinolones, aminoglycosides, and β-lactams-cause mitochondrial dysfunction and ROS overproduction in mammalian cells. We demonstrate that these bactericidal antibiotic-induced effects lead to oxidative damage to DNA, proteins, and membrane lipids. Mice treated with bactericidal antibiotics exhibited elevated oxidative stress markers in the blood, oxidative tissue damage, and up-regulated expression of key genes involved in antioxidant defense mechanisms, which points to the potential physiological relevance of these antibiotic effects. The deleterious effects of bactericidal antibiotics were alleviated in cell culture and in mice by the administration of the antioxidant N-acetyl-l-cysteine or prevented by preferential use of bacteriostatic antibiotics. This work highlights the role of antibiotics in the production of oxidative tissue damage in mammalian cells and presents strategies to mitigate or prevent the resulting damage, with the goal of improving the safety of antibiotic treatment in people.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

