STAT6 controls the number of regulatory T cells in vivo, thereby regulating allergic lung inflammation
- PMID: 23825312
- PMCID: PMC3735779
- DOI: 10.4049/jimmunol.1300486
STAT6 controls the number of regulatory T cells in vivo, thereby regulating allergic lung inflammation
Abstract
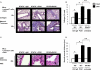
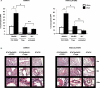
STAT6 plays a central role in IL-4-mediated allergic responses. Several studies indicate that regulatory T cells (Tregs) can be modulated by IL-4 in vitro. We previously showed that STAT6(-/-) mice are highly resistant to allergic lung inflammation even when wild-type Th2 effectors were provided and that they have increased numbers of Tregs. However, the role of STAT6 in modulating Tregs in vivo during allergic lung inflammation has not been thoroughly investigated. To examine Treg and STAT6 interaction during allergic inflammation, STAT6(-/-), STAT6xRAG2(-/-), and RAG2(-/-) mice were subjected to OVA sensitization and challenge following adoptive transfer of OVA-specific, wild-type Th2 effectors with or without prior Treg depletion/inactivation, using anti-CD25 (PC61). As expected, STAT6(-/-) mice were highly resistant to airway inflammation and remodeling. In contrast, allergic lung inflammation was partially restored in STAT6(-/-) mice treated with PC61 to levels observed in STAT6xRAG2(-/-) mice. In some cases, STAT6xRAG2(-/-) mice were also given natural Tregs along with Th2 effectors. Adoptive transfer of natural Tregs caused a substantial reduction in bronchoalveolar lavage eosinophil composition and suppressed airway remodeling and T cell migration into the lung in STAT6xRAG2(-/-) mice to levels comparable to those in STAT6(-/-) mice. These results demonstrate the STAT6-dependent suppression of Tregs in vivo to promote allergic airway inflammation.
Figures






References
-
- Perkins C, Wills-Karp M, Finkelman FD. IL-4 induces IL-13-independent allergic airway inflammation. The Journal of allergy and clinical immunology. 2006;118:410–419. - PubMed
-
- Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. The New England journal of medicine. 1992;326:298–304. - PubMed
-
- Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annual review of immunology. 1999;17:255–281. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

