Characterizing thalamo-cortical disturbances in schizophrenia and bipolar illness
- PMID: 23825317
- PMCID: PMC4224238
- DOI: 10.1093/cercor/bht165
Characterizing thalamo-cortical disturbances in schizophrenia and bipolar illness
Abstract
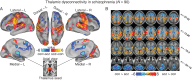
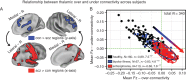
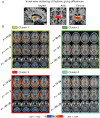
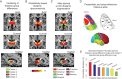
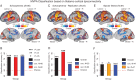
Schizophrenia is a devastating neuropsychiatric syndrome associated with distributed brain dysconnectivity that may involve large-scale thalamo-cortical systems. Incomplete characterization of thalamic connectivity in schizophrenia limits our understanding of its relationship to symptoms and to diagnoses with shared clinical presentation, such as bipolar illness, which may exist on a spectrum. Using resting-state functional magnetic resonance imaging, we characterized thalamic connectivity in 90 schizophrenia patients versus 90 matched controls via: (1) Subject-specific anatomically defined thalamic seeds; (2) anatomical and data-driven clustering to assay within-thalamus dysconnectivity; and (3) machine learning to classify diagnostic membership via thalamic connectivity for schizophrenia and for 47 bipolar patients and 47 matched controls. Schizophrenia analyses revealed functionally related disturbances: Thalamic over-connectivity with bilateral sensory-motor cortices, which predicted symptoms, but thalamic under-connectivity with prefrontal-striatal-cerebellar regions relative to controls, possibly reflective of sensory gating and top-down control disturbances. Clustering revealed that this dysconnectivity was prominent for thalamic nuclei densely connected with the prefrontal cortex. Classification and cross-diagnostic results suggest that thalamic dysconnectivity may be a neural marker for disturbances across diagnoses. Present findings, using one of the largest schizophrenia and bipolar neuroimaging samples to date, inform basic understanding of large-scale thalamo-cortical systems and provide vital clues about the complex nature of its disturbances in severe mental illness.
Keywords: bipolar illness; connectivity; resting state; schizophrenia; thalamus.
Published by Oxford University Press 2013. This work is written by (a) US Government employee(s) and is in the public domain in the US.
Figures

References
-
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. - PubMed
-
- Andreasen NC. The role of the thalamus in schizophrenia. Can J Psychiatry. 1997;42:27–33. - PubMed
-
- Andreasen NC, Arndt S, Swayze V, Cizadlo T, Flaum M, O'Leary D, Ehrhardt JC, Yuh WTC. Thalamic abnormalities in schizophrenia visualized through magnetic resonance image averaging. Science. 1994;266:294–298. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

