Unexpected effect of proton pump inhibitors: elevation of the cardiovascular risk factor asymmetric dimethylarginine
- PMID: 23825361
- PMCID: PMC3838201
- DOI: 10.1161/CIRCULATIONAHA.113.003602
Unexpected effect of proton pump inhibitors: elevation of the cardiovascular risk factor asymmetric dimethylarginine
Abstract
Background: Proton pump inhibitors (PPIs) are gastric acid-suppressing agents widely prescribed for the treatment of gastroesophageal reflux disease. Recently, several studies in patients with acute coronary syndrome have raised the concern that use of PPIs in these patients may increase their risk of major adverse cardiovascular events. The mechanism of this possible adverse effect is not known. Whether the general population might also be at risk has not been addressed.
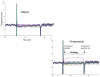
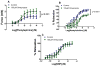
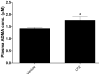
Methods and results: Plasma asymmetrical dimethylarginine (ADMA) is an endogenous inhibitor of nitric oxide synthase. Elevated plasma ADMA is associated with increased risk for cardiovascular disease, likely because of its attenuation of the vasoprotective effects of endothelial nitric oxide synthase. We find that PPIs elevate plasma ADMA levels and reduce nitric oxide levels and endothelium-dependent vasodilation in a murine model and ex vivo human tissues. PPIs increase ADMA because they bind to and inhibit dimethylarginine dimethylaminohydrolase, the enzyme that degrades ADMA.
Conclusions: We present a plausible biological mechanism to explain the association of PPIs with increased major adverse cardiovascular events in patients with unstable coronary syndromes. Of concern, this adverse mechanism is also likely to extend to the general population using PPIs. This finding compels additional clinical investigations and pharmacovigilance directed toward understanding the cardiovascular risk associated with the use of the PPIs in the general population.
Keywords: N,N dimethylarginine; dimethylarginine dimethylaminohydrolase; endothelium; nitric oxide; proton pump inhibitors.
Conflict of interest statement
Figures




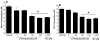
Comment in
-
Letter by Chyrchel et al regarding article, "unexpected effect of proton pump inhibitors: elevation of the cardiovascular risk factor asymmetric dimethylarginine".Circulation. 2014 Apr 1;129(13):e425. doi: 10.1161/CIRCULATIONAHA.113.006284. Circulation. 2014. PMID: 24687651 No abstract available.
-
Letter by Montenegro and Lundberg regarding article, "unexpected effect of proton pump inhibitors: elevation of the cardiovascular risk factor asymmetric dimethylarginine".Circulation. 2014 Apr 1;129(13):e426. doi: 10.1161/CIRCULATIONAHA.113.005585. Circulation. 2014. PMID: 24687652 No abstract available.
-
Letter by Pinheiro et al regarding article, "unexpected effect of proton pump inhibitors: elevation of the cardiovascular risk factor asymmetric dimethylarginine".Circulation. 2014 Apr 1;129(13):e427. doi: 10.1161/CIRCULATIONAHA.113.005307. Circulation. 2014. PMID: 24687653 No abstract available.
-
Response to letters regarding article, "unexpected effect of proton pump inhibitors: elevation of the cardiovascular risk factor asymmetric dimethylarginine".Circulation. 2014 Apr 1;129(13):e428. doi: 10.1161/CIRCULATIONAHA.114.009343. Circulation. 2014. PMID: 24687654 Free PMC article. No abstract available.
References
-
- Berardi RR, Welage LS. Proton-pump inhibitors in acid-related diseases. Am J Health Syst Pharm. 1998;55:2289–2298. - PubMed
-
- Welage LS, Berardi RR. Evaluation of omeprazole, lansoprazole, pantoprazole, and rabeprazole in the treatment of acid-related diseases. J Am Pharm Assoc (Wash) 2000;40:52–62. - PubMed
-
- Stedman CA, Barclay ML. Review article: comparison of the pharmacokinetics, acid suppression and efficacy of proton pump inhibitors. Aliment Pharmacol Ther. 2000;14:963–978. - PubMed
-
- Shin JM, Homerin M, Domagala F, Ficheux H, Sachs G. Characterization of the inhibitory activity of tenatoprazole on the gastric H+,K+-ATPase in vitro and in vivo. Biochem Pharmacol. 2006;71:837–849. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 HL100397/HL/NHLBI NIH HHS/United States
- RC2HL103400/HL/NHLBI NIH HHS/United States
- U54 HG004028/HG/NHGRI NIH HHS/United States
- 1U01HL100397/HL/NHLBI NIH HHS/United States
- MC_U120097118/MRC_/Medical Research Council/United Kingdom
- U54HG004028/HG/NHGRI NIH HHS/United States
- U54 LM008748/LM/NLM NIH HHS/United States
- U54LM008748/LM/NLM NIH HHS/United States
- K12HL087746/HL/NHLBI NIH HHS/United States
- RC2 HL103400/HL/NHLBI NIH HHS/United States
- K12 HL087746/HL/NHLBI NIH HHS/United States
- K01 HL118683/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

