Polymerase delta interacting protein 2 sustains vascular structure and function
- PMID: 23825363
- PMCID: PMC3837414
- DOI: 10.1161/ATVBAHA.113.301913
Polymerase delta interacting protein 2 sustains vascular structure and function
Erratum in
- Arterioscler Thromb Vasc Biol. 2013 Nov;33(11):e132
Abstract
Objective: On the basis of previous evidence that polymerase delta interacting protein 2 (Poldip2) increases reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 4 (Nox4) activity in vascular smooth muscle cells, we hypothesized that in vivo knockdown of Poldip2 would inhibit reactive oxygen species production and alter vascular function.
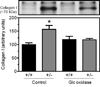
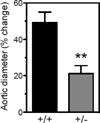
Approach and results: Because homozygous Poldip2 deletion is lethal, Poldip2(+/-) mice were used. Poldip2 mRNA and protein levels were reduced by ≈50% in Poldip2(+/-) aorta, with no change in p22phox, Nox1, Nox2, and Nox4 mRNAs. NADPH oxidase activity was also inhibited in Poldip2(+/-) tissue. Isolated aortas from Poldip2(+/-) mice demonstrated impaired phenylephrine and potassium chloride-induced contractions, increased stiffness, and reduced compliance associated with disruption of elastic lamellae and excessive extracellular matrix deposition. Collagen I secretion was elevated in cultured vascular smooth muscle cells from Poldip2(+/-) mice and restored by H2O2 supplementation, suggesting that this novel function of Poldip2 is mediated by reactive oxygen species. Furthermore, Poldip2(+/-) mice were protected against aortic dilatation in a model of experimental aneurysm, an effect consistent with increased collagen secretion.
Conclusions: Poldip2 knockdown reduces H2O2 production in vivo, leading to increases in extracellular matrix, greater vascular stiffness, and impaired agonist-mediated contraction. Thus, unaltered expression of Poldip2 is necessary for vascular integrity and function.
Keywords: Nox4; Poldip2; blood vessel; extracellular matrix; hydrogen peroxide.
Figures




References
-
- Laurent S, Alivon M, Beaussier H, Boutouyrie P. Aortic stiffness as a tissue biomarker for predicting future cardiovascular events in asymptomatic hypertensive subjects. Annals of medicine. 2012;44(Suppl 1):S93–S97. - PubMed
-
- Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. Journal of the American College of Cardiology. 2010;55:1318–1327. - PubMed
-
- Zhou RH, Vendrov AE, Tchivilev I, Niu XL, Molnar KC, Rojas M, Carter JD, Tong H, Stouffer GA, Madamanchi NR, Runge MS. Mitochondrial oxidative stress in aortic stiffening with age: The role of smooth muscle cell function. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:745–755. - PMC - PubMed
-
- Maiellaro-Rafferty K, Weiss D, Joseph G, Wan W, Gleason RL, Taylor WR. Catalase overexpression in aortic smooth muscle prevents pathological mechanical changes underlying abdominal aortic aneurysm formation. American journal of physiology. Heart and circulatory physiology. 2011;301:H355–H362. - PMC - PubMed
-
- Cucoranu I, Clempus R, Dikalova A, Phelan PJ, Ariyan S, Dikalov S, Sorescu D. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circulation research. 2005;97:900–907. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL095070/HL/NHLBI NIH HHS/United States
- HL070531/HL/NHLBI NIH HHS/United States
- HL058863/HL/NHLBI NIH HHS/United States
- HL095070/HL/NHLBI NIH HHS/United States
- HL38206/HL/NHLBI NIH HHS/United States
- R01 HL102167/HL/NHLBI NIH HHS/United States
- R37 HL038206/HL/NHLBI NIH HHS/United States
- I01 BX001910/BX/BLRD VA/United States
- DK074518/DK/NIDDK NIH HHS/United States
- R01 HL038206/HL/NHLBI NIH HHS/United States
- R01 HL070531/HL/NHLBI NIH HHS/United States
- R01 HL058863/HL/NHLBI NIH HHS/United States
- R01 DK074518/DK/NIDDK NIH HHS/United States
- HL102167/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

