Two-photon imaging of nonlinear glutamate release dynamics at bipolar cell synapses in the mouse retina
- PMID: 23825403
- PMCID: PMC3718381
- DOI: 10.1523/JNEUROSCI.1241-13.2013
Two-photon imaging of nonlinear glutamate release dynamics at bipolar cell synapses in the mouse retina
Abstract
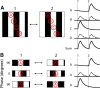
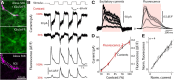
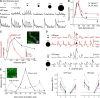
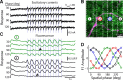
Alpha/Y-type retinal ganglion cells encode visual information with a receptive field composed of nonlinear subunits. This nonlinear subunit structure enhances sensitivity to patterns composed of high spatial frequencies. The Y-cell's subunits are the presynaptic bipolar cells, but the mechanism for the nonlinearity remains incompletely understood. We investigated the synaptic basis of the subunit nonlinearity by combining whole-cell recording of mouse Y-type ganglion cells with two-photon fluorescence imaging of a glutamate sensor (iGluSnFR) expressed on their dendrites and throughout the inner plexiform layer. A control experiment designed to assess iGluSnFR's dynamic range showed that fluorescence responses from Y-cell dendrites increased proportionally with simultaneously recorded excitatory current. Spatial resolution was sufficient to readily resolve independent release at intermingled ON and OFF bipolar terminals. iGluSnFR responses at Y-cell dendrites showed strong surround inhibition, reflecting receptive field properties of presynaptic release sites. Responses to spatial patterns located the origin of the Y-cell nonlinearity to the bipolar cell output, after the stage of spatial integration. The underlying mechanism differed between OFF and ON pathways: OFF synapses showed transient release and strong rectification, whereas ON synapses showed relatively sustained release and weak rectification. At ON synapses, the combination of fast release onset with slower release offset explained the nonlinear response of the postsynaptic ganglion cell. Imaging throughout the inner plexiform layer, we found transient, rectified release at the central-most levels, with increasingly sustained release near the borders. By visualizing glutamate release in real time, iGluSnFR provides a powerful tool for characterizing glutamate synapses in intact neural circuits.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
