Palmitoylation of amyloid precursor protein regulates amyloidogenic processing in lipid rafts
- PMID: 23825420
- PMCID: PMC3718372
- DOI: 10.1523/JNEUROSCI.4704-12.2013
Palmitoylation of amyloid precursor protein regulates amyloidogenic processing in lipid rafts
Abstract
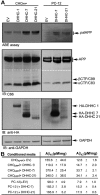
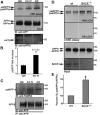
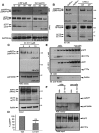
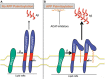
Brains of patients affected by Alzheimer's disease (AD) contain large deposits of aggregated amyloid β-protein (Aβ). Only a small fraction of the amyloid precursor protein (APP) gives rise to Aβ. Here, we report that ∼10% of APP undergoes a post-translational lipid modification called palmitoylation. We identified the palmitoylation sites in APP at Cys¹⁸⁶ and Cys¹⁸⁷. Surprisingly, point mutations introduced into these cysteines caused nearly complete ER retention of APP. Thus, either APP palmitoylation or disulfide bridges involving these Cys residues appear to be required for ER exit of APP. In later compartments, palmitoylated APP (palAPP) was specifically enriched in lipid rafts. In vitro BACE1 cleavage assays using cell or mouse brain lipid rafts showed that APP palmitoylation enhanced BACE1-mediated processing of APP. Interestingly, we detected an age-dependent increase in endogenous mouse brain palAPP levels. Overexpression of selected DHHC palmitoyl acyltransferases increased palmitoylation of APP and doubled Aβ production, while two palmitoylation inhibitors reduced palAPP levels and APP processing. We have found previously that acyl-coenzyme A:cholesterol acyltransferase (ACAT) inhibition led to impaired APP processing. Here we demonstrate that pharmacological inhibition or genetic inactivation of ACAT decrease lipid raft palAPP levels by up to 76%, likely resulting in impaired APP processing. Together, our results indicate that APP palmitoylation enhances amyloidogenic processing by targeting APP to lipid rafts and enhancing its BACE1-mediated cleavage. Thus, inhibition of palAPP formation by ACAT or specific palmitoylation inhibitors would appear to be a valid strategy for prevention and/or treatment of AD.
Figures





References
-
- Barnham KJ, McKinstry WJ, Multhaup G, Galatis D, Morton CJ, Curtain CC, Williamson NA, White AR, Hinds MG, Norton RS, Beyreuther K, Masters CL, Parker MW, Cappai R. Structure of the Alzheimer's disease amyloid precursor protein copper binding domain. A regulator of neuronal copper homeostasis. J Biol Chem. 2003;278:17401–17407. doi: 10.1074/jbc.M300629200. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
