Transcranial alternating current stimulation modulates large-scale cortical network activity by network resonance
- PMID: 23825429
- PMCID: PMC6618612
- DOI: 10.1523/JNEUROSCI.5867-12.2013
Transcranial alternating current stimulation modulates large-scale cortical network activity by network resonance
Abstract
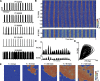
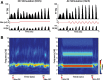
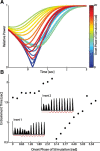
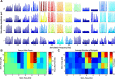
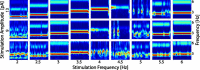
Transcranial direct current stimulation (tDCS) has emerged as a potentially safe and effective brain stimulation modality that alters cortical excitability by passing a small, constant electric current through the scalp. tDCS creates an electric field that weakly modulates the membrane voltage of a large number of cortical neurons. Recent human studies have suggested that sine-wave stimulation waveforms [transcranial alternating current stimulation (tACS)] represent a more targeted stimulation paradigm for the enhancement of cortical oscillations. Yet, the underlying mechanisms of how periodic, weak global perturbations alter the spatiotemporal dynamics of large-scale cortical network dynamics remain a matter of debate. Here, we simulated large-scale networks of spiking neuron models to address this question in endogenously rhythmic networks. We identified distinct roles of the depolarizing and hyperpolarizing phases of tACS in entrainment, which entailed moving network activity toward and away from a strong nonlinearity provided by the local excitatory coupling of pyramidal cells. Together, these mechanisms gave rise to resonance dynamics characterized by an Arnold tongue centered on the resonance frequency of the network. We then performed multichannel extracellular recordings of multiunit firing activity during tACS in anesthetized ferrets (Mustela putoris furo), a model species with a gyrencephalic brain, to verify that weak global perturbations can selectively enhance oscillations at the applied stimulation frequency. Together, these results provide a detailed mechanistic understanding of tACS at the level of large-scale network dynamics and support the future design of activity-dependent feedback tACS paradigms that dynamically tailor stimulation frequency to the spectral peak of ongoing brain activity.
Figures





Comment in
-
Modulation of cortical network activity by transcranial alternating current stimulation.J Neurosci. 2013 Nov 6;33(45):17551-2. doi: 10.1523/JNEUROSCI.3740-13.2013. J Neurosci. 2013. PMID: 24198344 Free PMC article. No abstract available.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
