Ferulic acid regulates the AKT/GSK-3β/CRMP-2 signaling pathway in a middle cerebral artery occlusion animal model
- PMID: 23825478
- PMCID: PMC3696626
- DOI: 10.5625/lar.2013.29.2.63
Ferulic acid regulates the AKT/GSK-3β/CRMP-2 signaling pathway in a middle cerebral artery occlusion animal model
Abstract
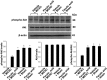
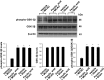
Ferulic acid, a component of the plants Angelica sinensis (Oliv.) Diels and Ligusticum chuanxiong Hort, exerts a neuroprotective effect by regulating various signaling pathways. This study showed that ferulic acid treatment prevents the injury-induced increase of collapsin response mediator protein 2 (CRMP-2) in focal cerebral ischemia. Glycogen synthase kinase-3β (GSK-3β) regulates CRMP-2 function through phosphorylation of CRMP-2. Moreover, the pro-apoptotic activity of GSK-3β is inactivated by phosphorylation by Akt. This study investigated whether ferulic acid modulates the expression of CRMP-2 and its upstream targets, Akt and GSK-3β, in focal cerebral ischemia. Male rats were treated immediately with ferulic acid (100 mg/kg, i.v.) or vehicle after middle cerebral artery occlusion (MCAO), and then cerebral cortices were collected 24 hr after MCAO. MCAO resulted in decreased levels of phospho-Akt and phospho-GSK-3β, while ferulic acid treatment prevented the decrease in the levels of these proteins. Moreover, phospho-CRMP-2 and CRMP-2 levels increased during MCAO, whereas ferulic acid attenuated these injury-induced increases. These results demonstrate that ferulic acid regulates the Akt/GSK-3β/CRMP-2 signaling pathway in focal cerebral ischemic injury, thereby protecting against brain injury.
Keywords: Akt; CRMP-2; Ferulic acid; GSK-3β; neuroprotection.
Figures


Similar articles
-
Resveratrol modulates the Akt/GSK-3β signaling pathway in a middle cerebral artery occlusion animal model.Lab Anim Res. 2019 Oct 15;35:18. doi: 10.1186/s42826-019-0019-8. eCollection 2019. Lab Anim Res. 2019. PMID: 32257906 Free PMC article.
-
Involvement of the Akt/GSK-3β/CRMP-2 pathway in axonal injury after hypoxic-ischemic brain damage in neonatal rat.Neuroscience. 2012 Aug 2;216:123-32. doi: 10.1016/j.neuroscience.2012.04.052. Epub 2012 Apr 30. Neuroscience. 2012. PMID: 22554777
-
Ferulic acid prevents the cerebral ischemic injury-induced decrease of Akt and Bad phosphorylation.Neurosci Lett. 2012 Jan 24;507(2):156-60. doi: 10.1016/j.neulet.2011.12.012. Epub 2011 Dec 17. Neurosci Lett. 2012. PMID: 22200499
-
Ferulic acid attenuates focal cerebral ischemia-induced decreases in p70S6 kinase and S6 phosphorylation.Neurosci Lett. 2013 Oct 25;555:7-11. doi: 10.1016/j.neulet.2013.09.001. Epub 2013 Sep 12. Neurosci Lett. 2013. PMID: 24036263
-
[Molecular mechanisms of neuronal polarity].Nihon Shinkei Seishin Yakurigaku Zasshi. 2005 Aug;25(4):169-74. Nihon Shinkei Seishin Yakurigaku Zasshi. 2005. PMID: 16190365 Review. Japanese.
Cited by
-
Efficacy and safety of probiotics in Parkinson's constipation: A systematic review and meta-analysis.Front Pharmacol. 2023 Jan 10;13:1007654. doi: 10.3389/fphar.2022.1007654. eCollection 2022. Front Pharmacol. 2023. PMID: 36703760 Free PMC article.
-
Resveratrol modulates the Akt/GSK-3β signaling pathway in a middle cerebral artery occlusion animal model.Lab Anim Res. 2019 Oct 15;35:18. doi: 10.1186/s42826-019-0019-8. eCollection 2019. Lab Anim Res. 2019. PMID: 32257906 Free PMC article.
-
Efficacy of ferulic acid in the treatment of acute ischemic stroke injury in rats: a systematic review and meta-analysis.Front Pharmacol. 2023 Oct 19;14:1278036. doi: 10.3389/fphar.2023.1278036. eCollection 2023. Front Pharmacol. 2023. PMID: 37927604 Free PMC article.
-
Ferulic acid prevents the injury-induced decrease of γ-enolase expression in brain tissue and HT22 cells.Lab Anim Res. 2014 Mar;30(1):8-13. doi: 10.5625/lar.2014.30.1.8. Epub 2014 Mar 24. Lab Anim Res. 2014. PMID: 24707299 Free PMC article.
-
The Protective Effects and Potential Mechanisms of Ligusticum chuanxiong: Focus on Anti-Inflammatory, Antioxidant, and Antiapoptotic Activities.Evid Based Complement Alternat Med. 2020 Oct 19;2020:8205983. doi: 10.1155/2020/8205983. eCollection 2020. Evid Based Complement Alternat Med. 2020. PMID: 33133217 Free PMC article. Review.
References
-
- Sultana R, Ravagna A, Mohmmad-Abdul H, Calabrese V, Butterfield DA. Ferulic acid ethyl ester protects neurons against amyloid beta-peptide(1-42)-induced oxidative stress and neurotoxicity: relationship to antioxidant activity. J Neurochem. 2005;92(4):749–758. - PubMed
-
- Srinivasan M, Sudheer AR, Pillai KR, Kumar PR, Sudhakaran PR, Menon VP. Influence of ferulic acid on gamma-radiation induced DNA damage, lipid peroxidation and antioxidant status in primary culture of isolated rat hepatocytes. Toxicology. 2006;228(2-3):249–258. - PubMed
-
- Kawabata K, Yamamoto T, Hara A, Shimizu M, Yamada Y, Matsunaga K, Tanaka T, Mori H. Modifying effects of ferulic acid on azoxymethane-induced colon carcinogenesis in F344 rats. Cancer Lett. 2000;157(1):15–21. - PubMed
-
- Ohnishi M, Matuo T, Tsuno T, Hosoda A, Nomura E, Taniguchi H, Sasaki H, Morishita H. Antioxidant activity and hypoglycemic effect of ferulic acid in STZ-induced diabetic mice and KK-Ay mice. Biofactors. 2004;21(1-4):315–319. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

