Multimodal Treatment Eliminates Cancer Stem Cells and Leads to Long-Term Survival in Primary Human Pancreatic Cancer Tissue Xenografts
- PMID: 23825539
- PMCID: PMC3688976
- DOI: 10.1371/journal.pone.0066371
Multimodal Treatment Eliminates Cancer Stem Cells and Leads to Long-Term Survival in Primary Human Pancreatic Cancer Tissue Xenografts
Abstract
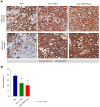
Purpose: In spite of intense research efforts, pancreatic ductal adenocarcinoma remains one of the most deadly malignancies in the world. We and others have previously identified a subpopulation of pancreatic cancer stem cells within the tumor as a critical therapeutic target and additionally shown that the tumor stroma represents not only a restrictive barrier for successful drug delivery, but also serves as a paracrine niche for cancer stem cells. Therefore, we embarked on a large-scale investigation on the effects of combining chemotherapy, hedgehog pathway inhibition, and mTOR inhibition in a preclinical mouse model of pancreatic cancer.
Experimental design: Prospective and randomized testing in a set of almost 200 subcutaneous and orthotopic implanted whole-tissue primary human tumor xenografts.
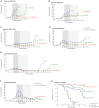
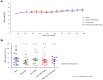
Results: The combined targeting of highly chemoresistant cancer stem cells as well as their more differentiated progenies, together with abrogation of the tumor microenvironment by targeting the stroma and enhancing tissue penetration of the chemotherapeutic agent translated into significantly prolonged survival in preclinical models of human pancreatic cancer. Most pronounced therapeutic effects were observed in gemcitabine-resistant patient-derived tumors. Intriguingly, the proposed triple therapy approach could be further enhanced by using a PEGylated formulation of gemcitabine, which significantly increased its bioavailability and tissue penetration, resulting in a further improved overall outcome.
Conclusions: This multimodal therapeutic strategy should be further explored in the clinical setting as its success may eventually improve the poor prognosis of patients with pancreatic ductal adenocarcinoma.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

