Regulation of Monocyte Adhesion and Migration by Nox4
- PMID: 23825596
- PMCID: PMC3688996
- DOI: 10.1371/journal.pone.0066964
Regulation of Monocyte Adhesion and Migration by Nox4
Abstract
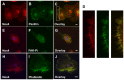
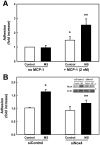
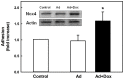
We showed that metabolic disorders promote thiol oxidative stress in monocytes, priming monocytes for accelerated chemokine-induced recruitment, and accumulation at sites of vascular injury and the progression of atherosclerosis. The aim of this study was to identify both the source of reactive oxygen species (ROS) responsible for thiol oxidation in primed and dysfunctional monocytes and the molecular mechanisms through which ROS accelerate the migration and recruitment of monocyte-derived macrophages. We found that Nox4, a recently identified NADPH oxidase in monocytes and macrophages, localized to focal adhesions and the actin cytoskeleton, and associated with phospho-FAK, paxillin, and actin, implicating Nox4 in the regulation of monocyte adhesion and migration. We also identified Nox4 as a new, metabolic stress-inducible source of ROS that controls actin S-glutathionylation and turnover in monocytes and macrophages, providing a novel mechanistic link between Nox4-derived H2O2 and monocyte adhesion and migration. Actin associated with Nox4 was S-glutathionylated, and Nox4 association with actin was enhanced in metabolically-stressed monocytes. Metabolic stress induced Nox4 and accelerated monocyte adhesion and chemotaxis in a Nox4-dependent mechanism. In conclusion, our data suggest that monocytic Nox4 is a central regulator of actin dynamics, and induction of Nox4 is the rate-limiting step in metabolic stress-induced monocyte priming and dysfunction associated with accelerated atherosclerosis and the progression of atherosclerotic plaques.
Conflict of interest statement
Figures


Similar articles
-
Protein Thiol Redox Signaling in Monocytes and Macrophages.Antioxid Redox Signal. 2016 Nov 20;25(15):816-835. doi: 10.1089/ars.2016.6697. Epub 2016 Jul 13. Antioxid Redox Signal. 2016. PMID: 27288099 Free PMC article. Review.
-
Ursolic acid protects monocytes against metabolic stress-induced priming and dysfunction by preventing the induction of Nox4.Redox Biol. 2014 Jan 11;2:259-66. doi: 10.1016/j.redox.2014.01.003. eCollection 2014. Redox Biol. 2014. PMID: 24494201 Free PMC article.
-
NADPH oxidase 4 mediates monocyte priming and accelerated chemotaxis induced by metabolic stress.Arterioscler Thromb Vasc Biol. 2012 Feb;32(2):415-26. doi: 10.1161/ATVBAHA.111.238899. Epub 2011 Nov 17. Arterioscler Thromb Vasc Biol. 2012. PMID: 22095986 Free PMC article.
-
Redox regulation of MAPK phosphatase 1 controls monocyte migration and macrophage recruitment.Proc Natl Acad Sci U S A. 2012 Oct 9;109(41):E2803-12. doi: 10.1073/pnas.1212596109. Epub 2012 Sep 18. Proc Natl Acad Sci U S A. 2012. PMID: 22991462 Free PMC article.
-
S-glutathionylation in monocyte and macrophage (dys)function.Int J Mol Sci. 2013 Jul 24;14(8):15212-32. doi: 10.3390/ijms140815212. Int J Mol Sci. 2013. PMID: 23887649 Free PMC article. Review.
Cited by
-
MicroRNA-146a decreases high glucose/thrombin-induced endothelial inflammation by inhibiting NAPDH oxidase 4 expression.Mediators Inflamm. 2014;2014:379537. doi: 10.1155/2014/379537. Epub 2014 Sep 14. Mediators Inflamm. 2014. PMID: 25298619 Free PMC article.
-
Protein S-Glutathionylation Mediates Macrophage Responses to Metabolic Cues from the Extracellular Environment.Antioxid Redox Signal. 2016 Nov 20;25(15):836-851. doi: 10.1089/ars.2015.6531. Epub 2016 May 17. Antioxid Redox Signal. 2016. PMID: 26984580 Free PMC article.
-
Protein Thiol Redox Signaling in Monocytes and Macrophages.Antioxid Redox Signal. 2016 Nov 20;25(15):816-835. doi: 10.1089/ars.2016.6697. Epub 2016 Jul 13. Antioxid Redox Signal. 2016. PMID: 27288099 Free PMC article. Review.
-
Redox Homeostasis in Thyroid Cancer: Implications in Na+/I- Symporter (NIS) Regulation.Int J Mol Sci. 2022 May 30;23(11):6129. doi: 10.3390/ijms23116129. Int J Mol Sci. 2022. PMID: 35682803 Free PMC article. Review.
-
Age-related Beta-synuclein Alters the p53/Mdm2 Pathway and Induces the Apoptosis of Brain Microvascular Endothelial Cells In Vitro.Cell Transplant. 2018 May;27(5):796-813. doi: 10.1177/0963689718755706. Epub 2018 May 29. Cell Transplant. 2018. PMID: 29808713 Free PMC article.
References
-
- Libby P (2002) Inflammation in atherosclerosis. Nature 420: 868–874. - PubMed
-
- Ross R (1999) Atherosclerosis – an inflammatory disease. N Engl J Med 340: 115–126. - PubMed
-
- Wolin MS, Ahmad M, Gupte SA (2005) Oxidant and redox signaling in vascular oxygen sensing mechanisms: basic concepts, current controversies, and potential importance of cytosolic NADPH. Am J Physiol Lung Cell Mol Physiol 289: L159–173. - PubMed
-
- Forman HJ, Torres M, Fukuto J (2002) Redox signaling. Mol Cell Biochem 234–235: 49–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

