Interleukin-21 enhances rituximab activity in a cynomolgus monkey model of B cell depletion and in mouse B cell lymphoma models
- PMID: 23825648
- PMCID: PMC3692496
- DOI: 10.1371/journal.pone.0067256
Interleukin-21 enhances rituximab activity in a cynomolgus monkey model of B cell depletion and in mouse B cell lymphoma models
Abstract
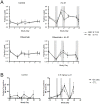
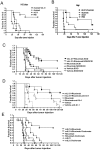
Rituximab, a monoclonal antibody targeting CD20 on B cells, is currently used to treat many subtypes of B cell lymphomas. However, treatment is not curative and response rates are variable. Recombinant interleukin-21 (rIL-21) is a cytokine that enhances immune effector function and affects both primary and transformed B cell differentiation. We hypothesized that the combination of rIL-21 plus rituximab would be a more efficacious treatment for B cell malignancies than rituximab alone. We cultured human and cynomolgus monkey NK cells with rIL-21 and found that their activity was increased and proteins associated with antibody dependent cytotoxicity were up-regulated. Studies in cynomolgus monkeys modeled the effects of rIL-21 on rituximab activity against CD20 B cells. In these studies, rIL-21 activated innate immune effectors, increased ADCC and mobilized B cells into peripheral blood. When rIL-21 was combined with rituximab, deeper and more durable B cell depletion was observed. In another series of experiments, IL-21 was shown to have direct antiproliferative activity against a subset of human lymphoma cell lines, and combination of murine IL-21 with rituximab yielded significant survival benefits over either agent alone in xenogeneic mouse tumor models of disseminated lymphoma. Therefore, our results do suggest that the therapeutic efficacy of rituximab may be improved when used in combination with rIL-21.
Conflict of interest statement
Figures






References
-
- Asao H, Okuyama C, Kumaki S, Ishii N, Tsuchiya S, et al. (2001) Cutting edge: the common gamma-chain is an indispensable subunit of the IL-21 receptor complex. J Immunol 167: 1–5. - PubMed
-
- Habib T, Senadheera S, Weinberg K, Kaushansky K (2002) The common gamma chain (gamma c) is a required signaling component of the IL-21 receptor and supports IL-21-induced cell proliferation via JAK3. Biochemistry 41: 8725–8731. - PubMed
-
- Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, et al. (2000) Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature 408: 57–63. - PubMed
-
- Ettinger R, Sims GP, Fairhurst AM, Robbins R, da Silva YS, et al. (2005) IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J Immunol 175: 7867–7879. - PubMed
-
- Jin H, Carrio R, Yu A, Malek TR (2004) Distinct activation signals determine whether IL-21 induces B cell costimulation, growth arrest, or Bim-dependent apoptosis. J Immunol 173: 657–665. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

