Alternative complement pathway deficiency ameliorates chronic smoke-induced functional and morphological ocular injury
- PMID: 23825688
- PMCID: PMC3692454
- DOI: 10.1371/journal.pone.0067894
Alternative complement pathway deficiency ameliorates chronic smoke-induced functional and morphological ocular injury
Abstract
Background: Age-related macular degeneration (AMD), a complex disease involving genetic variants and environmental insults, is among the leading causes of blindness in Western populations. Genetic and histologic evidence implicate the complement system in AMD pathogenesis; and smoking is the major environmental risk factor associated with increased disease risk. Although previous studies have demonstrated that cigarette smoke exposure (CE) causes retinal pigment epithelium (RPE) defects in mice, and smoking leads to complement activation in patients, it is unknown whether complement activation is causative in the development of CE pathology; and if so, which complement pathway is required.
Methods: Mice were exposed to cigarette smoke or clean, filtered air for 6 months. The effects of CE were analyzed in wildtype (WT) mice or mice without a functional complement alternative pathway (AP; CFB(-/-) ) using molecular, histological, electrophysiological, and behavioral outcomes.
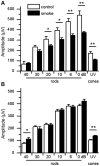
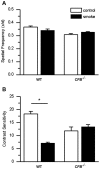
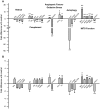
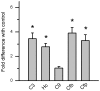
Results: CE in WT mice exhibited a significant reduction in function of both rods and cones as determined by electroretinography and contrast sensitivity measurements, concomitant with a thinning of the nuclear layers as measured by SD-OCT imaging and histology. Gene expression analyses suggested that alterations in both photoreceptors and RPE/choroid might contribute to the observed loss of function, and visualization of complement C3d deposition implies the RPE/Bruch's membrane (BrM) complex as the target of AP activity. RPE/BrM alterations include an increase in mitochondrial size concomitant with an apical shift in mitochondrial distribution within the RPE and a thickening of BrM. CFB(-/-) mice were protected from developing these CE-mediated alterations.
Conclusions: Taken together, these findings provide clear evidence that ocular pathology generated in CE mice is dependent on complement activation and requires the AP. Identifying animal models with RPE/BrM damage and verifying which aspects of pathology are dependent upon complement activation is essential for developing novel complement-based treatment approaches for the treatment of AMD.
Conflict of interest statement
Figures



References
-
- Freund KB, Zweifel SA, Engelbert M (2010) Do we need a new classification for choroidal neovascularization in age-related macular degeneration? Retina 30: 1333–1349. - PubMed
-
- Brown MM, Brown GC, Stein JD, Roth Z, Campanella J, et al. (2005) Age-related macular degeneration: economic burden and value-based medicine analysis. Can J Ophthalmol 40: 277–287. - PubMed
-
- Edwards AO, Ritter R, 3rd, Abel KJ, Manning A, Panhuysen C, et al (2005) Complement factor H polymorphism and age-related macular degeneration. Science 308: 421–424. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

