Deformable M-Reps for 3D Medical Image Segmentation
- PMID: 23825898
- PMCID: PMC3697155
- DOI: 10.1023/a:1026313132218
Deformable M-Reps for 3D Medical Image Segmentation
Abstract
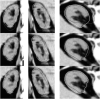
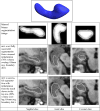
M-reps (formerly called DSLs) are a multiscale medial means for modeling and rendering 3D solid geometry. They are particularly well suited to model anatomic objects and in particular to capture prior geometric information effectively in deformable models segmentation approaches. The representation is based on figural models, which define objects at coarse scale by a hierarchy of figures - each figure generally a slab representing a solid region and its boundary simultaneously. This paper focuses on the use of single figure models to segment objects of relatively simple structure. A single figure is a sheet of medial atoms, which is interpolated from the model formed by a net, i.e., a mesh or chain, of medial atoms (hence the name m-reps), each atom modeling a solid region via not only a position and a width but also a local figural frame giving figural directions and an object angle between opposing, corresponding positions on the boundary implied by the m-rep. The special capability of an m-rep is to provide spatial and orientational correspondence between an object in two different states of deformation. This ability is central to effective measurement of both geometric typicality and geometry to image match, the two terms of the objective function optimized in segmentation by deformable models. The other ability of m-reps central to effective segmentation is their ability to support segmentation at multiple levels of scale, with successively finer precision. Objects modeled by single figures are segmented first by a similarity transform augmented by object elongation, then by adjustment of each medial atom, and finally by displacing a dense sampling of the m-rep implied boundary. While these models and approaches also exist in 2D, we focus on 3D objects. The segmentation of the kidney from CT and the hippocampus from MRI serve as the major examples in this paper. The accuracy of segmentation as compared to manual, slice-by-slice segmentation is reported.
Figures
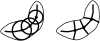



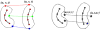
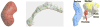









References
-
- Amenta N, Bern M, Kamvysselis M. A New Voronoi-Based Surface Reconstruction Algorithm. Computer Graphics; Proceedings, Annual Conference Series; 1998; ACM SIGGRAPH; 1998. pp. 415–422.
-
- Attali D, Sanniti di Baja G, Thiel E. Skeleton simplification through non significant branch removal. Image Processing and Communications. 1997;3(3-4):63–72.
-
- Bittar E, Tsingos N, Gascuel M. Automatic Reconstruction of Unstructured 3D Data: Combining a Medial Axis and Implicit Surfaces. Computer Graphics Forum (Eurographics '95) 1995;14(3):457–468.
-
- Blum H. A transformation for extracting new descriptors of shape. In: Wathen-Dunn W, editor. Models for the Perception of Speech and Visual Form. MIT Press; Cambridge MA: 1967. pp. 363–380.
-
- Burbeck CA, Pizer SM, Morse BS, Ariely D, Zauberman G, Rolland J. Linking object boundaries at scale: a common mechanism for size and shape judgments. University of North Carolina Computer Science Department technical report TR94-041. Vision Research. 1996;36(3):361–372. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
