Novel immunomodulators from hard ticks selectively reprogramme human dendritic cell responses
- PMID: 23825947
- PMCID: PMC3695081
- DOI: 10.1371/journal.ppat.1003450
Novel immunomodulators from hard ticks selectively reprogramme human dendritic cell responses
Abstract
Hard ticks subvert the immune responses of their vertebrate hosts in order to feed for much longer periods than other blood-feeding ectoparasites; this may be one reason why they transmit perhaps the greatest diversity of pathogens of any arthropod vector. Tick-induced immunomodulation is mediated by salivary components, some of which neutralise elements of innate immunity or inhibit the development of adaptive immunity. As dendritic cells (DC) trigger and help to regulate adaptive immunity, they are an ideal target for immunomodulation. However, previously described immunoactive components of tick saliva are either highly promiscuous in their cellular and molecular targets or have limited effects on DC. Here we address the question of whether the largest and globally most important group of ticks (the ixodid metastriates) produce salivary molecules that specifically modulate DC activity. We used chromatography to isolate a salivary gland protein (Japanin) from Rhipicephalus appendiculatus ticks. Japanin was cloned, and recombinant protein was produced in a baculoviral expression system. We found that Japanin specifically reprogrammes DC responses to a wide variety of stimuli in vitro, radically altering their expression of co-stimulatory and co-inhibitory transmembrane molecules (measured by flow cytometry) and their secretion of pro-inflammatory, anti-inflammatory and T cell polarising cytokines (assessed by Luminex multiplex assays); it also inhibits the differentiation of DC from monocytes. Sequence alignments and enzymatic deglycosylation revealed Japanin to be a 17.7 kDa, N-glycosylated lipocalin. Using molecular cloning and database searches, we have identified a group of homologous proteins in R. appendiculatus and related species, three of which we have expressed and shown to possess DC-modulatory activity. All data were obtained using DC generated from at least four human blood donors, with rigorous statistical analysis. Our results suggest a previously unknown mechanism for parasite-induced subversion of adaptive immunity, one which may also facilitate pathogen transmission.
Conflict of interest statement
I have read the journal's policy and have the following conflicts. Some of the data in this publication have been used in the patent applications WO2010/032008 and WO2011/117582 which are owned by the Natural Environment Research Council (NERC) in agreement with the University of Oxford, and were licenced to IXO Therapeutics Ltd. until 31st Dec 2012; the licence has now expired and there are currently no commercial activities relating to these patents. SGP, CK, PAN and JMA were formerly shareholders of, and SGP, GCP and JMA were consultants to, IXO Therapeutics Ltd.; this spin-out company is no longer operational. This does not alter our adherence to all PLoS Pathogens policies on sharing data and materials.
Figures
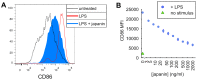
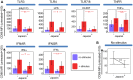
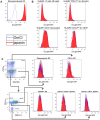
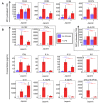
 p<0.1, NS p>0.05.
p<0.1, NS p>0.05.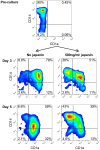

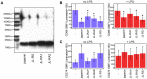

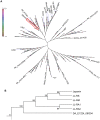
References
-
- Kaufman WR (2007) Gluttony and sex in female ixodid ticks: how do they compare to other blood-sucking arthropods? Journal of insect physiology 53: 264–273 doi:10.1016/j.jinsphys.2006.10.004 - DOI - PubMed
-
- Marquardt WH, editor (2004) Biology of Disease Vectors. 2nd ed. Burlington: Academic Press. 786 p.
-
- Bianchi ME (2007) DAMPs, PAMPs and alarmins: all we need to know about danger. Journal of leukocyte biology 81: 1–5 doi:10.1189/jlb.0306164 - DOI - PubMed
-
- Paesen GC, Adams PL, Harlos K, Nuttall PA, Stuart DI (1999) Tick histamine-binding proteins: isolation, cloning, and three-dimensional structure. Molecular cell 3: 661–671. - PubMed
-
- Valenzuela JG, Charlab R, Mather TN, Ribeiro JM (2000) Purification, cloning, and expression of a novel salivary anticomplement protein from the tick, Ixodes scapularis. The Journal of biological chemistry 275: 18717–18723 doi:10.1074/jbc.M001486200 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

