Transcriptome-wide mapping of 5-methylcytidine RNA modifications in bacteria, archaea, and yeast reveals m5C within archaeal mRNAs
- PMID: 23825970
- PMCID: PMC3694839
- DOI: 10.1371/journal.pgen.1003602
Transcriptome-wide mapping of 5-methylcytidine RNA modifications in bacteria, archaea, and yeast reveals m5C within archaeal mRNAs
Abstract
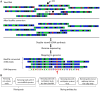
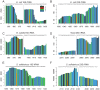
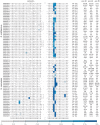
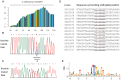
The presence of 5-methylcytidine (m(5)C) in tRNA and rRNA molecules of a wide variety of organisms was first observed more than 40 years ago. However, detection of this modification was limited to specific, abundant, RNA species, due to the usage of low-throughput methods. To obtain a high resolution, systematic, and comprehensive transcriptome-wide overview of m(5)C across the three domains of life, we used bisulfite treatment on total RNA from both gram positive (B. subtilis) and gram negative (E. coli) bacteria, an archaeon (S. solfataricus) and a eukaryote (S. cerevisiae), followed by massively parallel sequencing. We were able to recover most previously documented m(5)C sites on rRNA in the four organisms, and identified several novel sites in yeast and archaeal rRNAs. Our analyses also allowed quantification of methylated m(5)C positions in 64 tRNAs in yeast and archaea, revealing stoichiometric differences between the methylation patterns of these organisms. Molecules of tRNAs in which m(5)C was absent were also discovered. Intriguingly, we detected m(5)C sites within archaeal mRNAs, and identified a consensus motif of AUCGANGU that directs methylation in S. solfataricus. Our results, which were validated using m(5)C-specific RNA immunoprecipitation, provide the first evidence for mRNA modifications in archaea, suggesting that this mode of post-transcriptional regulation extends beyond the eukaryotic domain.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Basu R, Zhang LF (2011) X chromosome inactivation: a silence that needs to be broken. Genesis 49: 821–834. - PubMed
-
- Rigal M, Mathieu O (2011) A “mille-feuille” of silencing: epigenetic control of transposable elements. Biochim Biophys Acta 1809: 452–458. - PubMed
-
- Motorin Y, Helm M (2010) tRNA stabilization by modified nucleotides. Biochemistry 49: 4934–4944. - PubMed
-
- Squires JE, Preiss T (2010) Function and detection of 5-methylcytosine in eukaryotic RNA. Epigenomics 2: 709–715. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

