Discovery of a novel dual fungal CYP51/human 5-lipoxygenase inhibitor: implications for anti-fungal therapy
- PMID: 23826084
- PMCID: PMC3691235
- DOI: 10.1371/journal.pone.0065928
Discovery of a novel dual fungal CYP51/human 5-lipoxygenase inhibitor: implications for anti-fungal therapy
Abstract
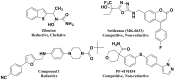
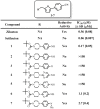
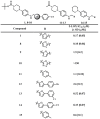
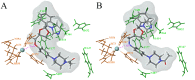
We report the discovery of a novel dual inhibitor targeting fungal sterol 14α-demethylase (CYP51 or Erg11) and human 5-lipoxygenase (5-LOX) with improved potency against 5-LOX due to its reduction of the iron center by its phenylenediamine core. A series of potent 5-LOX inhibitors containing a phenylenediamine core, were synthesized that exhibit nanomolar potency and >30-fold selectivity against the LOX paralogs, platelet-type 12-human lipoxygenase, reticulocyte 15-human lipoxygenase type-1, and epithelial 15-human lipoxygenase type-2, and >100-fold selectivity against ovine cyclooxygenase-1 and human cyclooxygnease-2. The phenylenediamine core was then translated into the structure of ketoconazole, a highly effective anti-fungal medication for seborrheic dermatitis, to generate a novel compound, ketaminazole. Ketaminazole was found to be a potent dual inhibitor against human 5-LOX (IC50 = 700 nM) and CYP51 (IC50 = 43 nM) in vitro. It was tested in whole blood and found to down-regulate LTB4 synthesis, displaying 45% inhibition at 10 µM. In addition, ketaminazole selectively inhibited yeast CYP51 relative to human CYP51 by 17-fold, which is greater selectivity than that of ketoconazole and could confer a therapeutic advantage. This novel dual anti-fungal/anti-inflammatory inhibitor could potentially have therapeutic uses against fungal infections that have an anti-inflammatory component.
Conflict of interest statement
Figures



Similar articles
-
Azole Antifungal Sensitivity of Sterol 14α-Demethylase (CYP51) and CYP5218 from Malassezia globosa.Sci Rep. 2016 Jun 13;6:27690. doi: 10.1038/srep27690. Sci Rep. 2016. PMID: 27291783 Free PMC article.
-
Crystal Structure of the New Investigational Drug Candidate VT-1598 in Complex with Aspergillus fumigatus Sterol 14α-Demethylase Provides Insights into Its Broad-Spectrum Antifungal Activity.Antimicrob Agents Chemother. 2017 Jun 27;61(7):e00570-17. doi: 10.1128/AAC.00570-17. Print 2017 Jul. Antimicrob Agents Chemother. 2017. PMID: 28461309 Free PMC article.
-
Tepoxalin: a dual cyclooxygenase/5-lipoxygenase inhibitor of arachidonic acid metabolism with potent anti-inflammatory activity and a favorable gastrointestinal profile.J Pharmacol Exp Ther. 1994 Dec;271(3):1399-408. J Pharmacol Exp Ther. 1994. PMID: 7996452
-
Fungal cytochrome P450 sterol 14α-demethylase (CYP51) and azole resistance in plant and human pathogens.Appl Microbiol Biotechnol. 2012 Aug;95(4):825-40. doi: 10.1007/s00253-012-4195-9. Epub 2012 Jun 12. Appl Microbiol Biotechnol. 2012. PMID: 22684327 Review.
-
Dual inhibitors of cyclooxygenase and 5-lipoxygenase. A new avenue in anti-inflammatory therapy?Biochem Pharmacol. 2001 Dec 1;62(11):1433-8. doi: 10.1016/s0006-2952(01)00747-x. Biochem Pharmacol. 2001. PMID: 11728379 Review.
Cited by
-
Next-generation antifungal drugs: Mechanisms, efficacy, and clinical prospects.Acta Pharm Sin B. 2025 Aug;15(8):3852-3887. doi: 10.1016/j.apsb.2025.06.013. Epub 2025 Jun 23. Acta Pharm Sin B. 2025. PMID: 40893690 Free PMC article. Review.
-
Versatile biocatalysis of fungal cytochrome P450 monooxygenases.Microb Cell Fact. 2016 Jul 18;15(1):125. doi: 10.1186/s12934-016-0523-6. Microb Cell Fact. 2016. PMID: 27431996 Free PMC article. Review.
-
Computational Analysis of LOX1 Inhibition Identifies Descriptors Responsible for Binding Selectivity.ACS Omega. 2018 Feb 28;3(2):2261-2272. doi: 10.1021/acsomega.7b01622. Epub 2018 Feb 26. ACS Omega. 2018. PMID: 30023828 Free PMC article.
-
Azole Antifungal Sensitivity of Sterol 14α-Demethylase (CYP51) and CYP5218 from Malassezia globosa.Sci Rep. 2016 Jun 13;6:27690. doi: 10.1038/srep27690. Sci Rep. 2016. PMID: 27291783 Free PMC article.
-
Ru(II) photocages enable precise control over enzyme activity with red light.Nat Commun. 2022 Jun 25;13(1):3636. doi: 10.1038/s41467-022-31269-5. Nat Commun. 2022. PMID: 35752630 Free PMC article.
References
-
- Rubin P, Mollison KW (2007) Pharmacotherapy of diseases mediated by 5-lipoxygenase pathway eicosanoids. Prostaglandins Other Lipid Mediat 83: 188–197. - PubMed
-
- O'Byrne PM, Israel E, Drazen JM (1997) Antileukotrienes in the Treatment of Asthma. Annals of Internal Medicine 127: 472–480. - PubMed
-
- Rådmark OP (2000) The Molecular Biology and Regulation of 5-Lipoxygenase. American Journal of Respiratory and Critical Care Medicine 161: S11–S15. - PubMed
-
- Ford-Hutchinson AW, Gresser M, Young RN (1994) 5-Lipoxygenase. Annu Rev Biochem 63: 383–417. - PubMed
-
- Werz O, Steinhilber D (2006) Therapeutic options for 5-lipoxygenase inhibitors. Pharmacology & Therapeutics 112: 701–718. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

