EffiCiency and Safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study
- PMID: 23826093
- PMCID: PMC3691171
- DOI: 10.1371/journal.pone.0066317
EffiCiency and Safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study
Erratum in
- PLoS One. 2014;9(1). doi:10.1371/annotation/e12c22d3-a42b-455d-9100-6c7ee45d58d0
Abstract
Background: Electronic cigarettes (e-cigarettes) are becoming increasingly popular with smokers worldwide. Users report buying them to help quit smoking, to reduce cigarette consumption, to relieve tobacco withdrawal symptoms, and to continue having a 'smoking' experience, but with reduced health risks. Research on e-cigarettes is urgently needed in order to ensure that the decisions of regulators, healthcare providers and consumers are based on science. Methods ECLAT is a prospective 12-month randomized, controlled trial that evaluates smoking reduction/abstinence in 300 smokers not intending to quit experimenting two different nicotine strengths of a popular e-cigarette model ('Categoria'; Arbi Group Srl, Italy) compared to its non-nicotine choice. GroupA (n = 100) received 7.2 mg nicotine cartridges for 12 weeks; GroupB (n = 100), a 6-week 7.2 mg nicotine cartridges followed by a further 6-week 5.4 mg nicotine cartridges; GroupC (n = 100) received no-nicotine cartridges for 12 weeks. The study consisted of nine visits during which cig/day use and exhaled carbon monoxide (eCO) levels were measured. Smoking reduction and abstinence rates were calculated. Adverse events and product preferences were also reviewed.
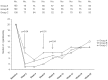
Results: Declines in cig/day use and eCO levels were observed at each study visits in all three study groups (p<0.001 vs baseline), with no consistent differences among study groups. Smoking reduction was documented in 22.3% and 10.3% at week-12 and week-52 respectively. Complete abstinence from tobacco smoking was documented in 10.7% and 8.7% at week-12 and week-52 respectively. A substantial decrease in adverse events from baseline was observed and withdrawal symptoms were infrequently reported during the study. Participants' perception and acceptance of the product under investigation was satisfactory.
Conclusion: In smokers not intending to quit, the use of e-cigarettes, with or without nicotine, decreased cigarette consumption and elicited enduring tobacco abstinence without causing significant side effects.
Trial registration: ClinicalTrials.gov NCT01164072 NCT01164072.
Conflict of interest statement
Figures






Comment in
-
E-cigarettes as tobacco harm-reduction tool: a promise or peril?Natl Med J India. 2013 Nov-Dec;26(6):340-1. Natl Med J India. 2013. PMID: 25073992 No abstract available.
References
-
- World Health Organization (2008) Report on the global tobacco epidemic. www.who.int/tobacco/mpower/2008/en/index.html
-
- The health benefits of smoking cessation (1990) In. Edited by Services UDoHaH; USA, US Department of Health and Human Services, Public Health Service, Centers for Disease Control, Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health.
-
- World Health Organization (WHO), WHO Framework Convention on Tobacco Control. ISBN: 9241591013.
-
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz N, et al. (2008). Treating tobacco use and dependence: 2008 Update. Rockville (MD): U.S. Department of Health and Human Services.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

