HSPB1 facilitates the formation of non-centrosomal microtubules
- PMID: 23826100
- PMCID: PMC3691211
- DOI: 10.1371/journal.pone.0066541
HSPB1 facilitates the formation of non-centrosomal microtubules
Abstract
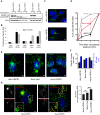
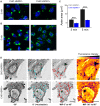
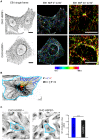
The remodeling capacity of microtubules (MT) is essential for their proper function. In mammals, MTs are predominantly formed at the centrosome, but can also originate from non-centrosomal sites, a process that is still poorly understood. We here show that the small heat shock protein HSPB1 plays a role in the control of non-centrosomal MT formation. The HSPB1 expression level regulates the balance between centrosomal and non-centrosomal MTs. The HSPB1 protein can be detected specifically at sites of de novo forming non-centrosomal MTs, while it is absent from the centrosomes. In addition, we show that HSPB1 binds preferentially to the lattice of newly formed MTs in vitro, suggesting that its function occurs by stabilizing MT seeds. Our findings open new avenues for the understanding of the role of HSPB1 in the development, maintenance and protection of cells with specialized non-centrosomal MT arrays.
Conflict of interest statement
Figures


References
-
- Sun Y, Macrae TH (2005) The small heat shock proteins and their role in human disease. FEBS J 272: 2613–2627. - PubMed
-
- Evgrafov OV, Mersiyanova I, Irobi J, Van Den Bosch L, Dierick I, et al. (2004) Mutant small heat-shock protein 27 causes axonal Charcot-Marie-Tooth disease and distal hereditary motor neuropathy. Nat Genet 36: 602–606. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

