'Cand. Actinochlamydia clariae' gen. nov., sp. nov., a unique intracellular bacterium causing epitheliocystis in catfish (Clarias gariepinus) in Uganda
- PMID: 23826156
- PMCID: PMC3691252
- DOI: 10.1371/journal.pone.0066840
'Cand. Actinochlamydia clariae' gen. nov., sp. nov., a unique intracellular bacterium causing epitheliocystis in catfish (Clarias gariepinus) in Uganda
Abstract
Background and objectives: Epitheliocystis, caused by bacteria infecting gill epithelial cells in fish, is common among a large range of fish species in both fresh- and seawater. The aquaculture industry considers epitheliocystis an important problem. It affects the welfare of the fish and the resulting gill disease may lead to mortalities. In a culture facility in Kampala, Uganda, juveniles of the African sharptooth catfish (Clarias gariepinus) was observed swimming in the surface, sometimes belly up, showing signs of respiratory problems. Histological examination of gill tissues from this fish revealed large amounts of epitheliocysts, and also presence of a few Ichthyobodo sp. and Trichodina sp.
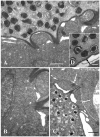
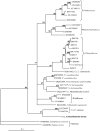
Methods and results: Sequencing of the epitheliocystis bacterium 16S rRNA gene shows 86.3% similarity with Candidatus Piscichlamydia salmonis causing epitheliocystis in Atlantic salmon (Salmo salar). Transmission electron microscopy showed that the morphology of the developmental stages of the bacterium is similar to that of members of the family Chlamydiaceae. The similarity of the bacterium rRNA gene sequences compared with other chlamydia-like bacteria ranged between 80.5% and 86.3%. Inclusions containing this new bacterium have tubules/channels (termed actinae) that are radiating from the inclusion membrane and opening on the cell surface or in neighbouring cells.
Conclusions: Radiation of tubules/channels (actinae) from the inclusion membrane has never been described in any of the other members of Chlamydiales. It seems to be a completely new character and an apomorphy. We propose the name Candidatus Actinochlamydia clariae gen. nov., sp. nov. (Actinochlamydiaceae fam. nov., order Chlamydiales, phylum Chlamydiae) for this new agent causing epitheliocystis in African sharptooth catfish.
Conflict of interest statement
Figures








References
-
- Plehn M (1920) Neue Parasiten in Haut und Kiemen von Fishen. Ichthyochytrium und Mucophilus. Zentbl Bakteriolog P 85: 275–281.
-
- Hoffman GL, Dunbar CE, Wolf K, Zwillenberg LO (1969) Epitheliocystis, a new infectious disease of the bluegill (Lepomis macrochirus). Antonie van Leeuwenhoek 35: 146–158. - PubMed
-
- Moulder JW (1964) The psittacosis group as bacteria. Wiley, NY.
-
- Storz J, Page LA (1971) Taxonomy of the Chlamydiae: reasons for classifying Organisms of the Genus Chlamydia, Family Chlamydiaceae, in a Separate Order, Chlamydiales ord. nov. Int J Syst Evol Microbiol 21(4): 332–334.
-
- Garrity GM, Holt JG (2001) The Road Map to the Manual. In: Bergey’s Manual of Systematic Bacteriology, 2nd edn, vol 1, The Archaea and the Deeply Branching and Phototrophic Bacteria (ed Boone, Castenholz and Garrity). Springer, NY: 119–166.
Publication types
MeSH terms
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

