The Time Course of Gene Expression during Reactive Gliosis in the Optic Nerve
- PMID: 23826199
- PMCID: PMC3694957
- DOI: 10.1371/journal.pone.0067094
The Time Course of Gene Expression during Reactive Gliosis in the Optic Nerve
Abstract
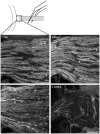
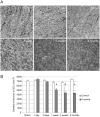
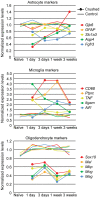
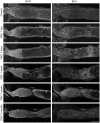
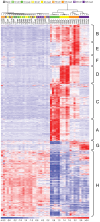
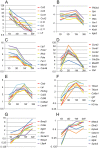
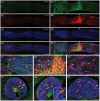
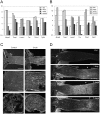
Reactive gliosis is a complex process that involves changes in gene expression and morphological remodeling. The mouse optic nerve, where astrocytes, microglia and oligodendrocytes interact with retinal ganglion cell axons and each other, is a particularly suitable model for studying the molecular mechanisms of reactive gliosis. We triggered gliosis at the mouse optic nerve head by retro orbital nerve crush. We followed the expression profiles of 14,000 genes from 1 day to 3 months, as the optic nerve formed a glial scar. The transcriptome showed profound changes. These were greatest shortly after injury; the numbers of differentially regulated genes then dropped, returning nearly to resting levels by 3 months. Different genes were modulated with very different time courses, and functionally distinct groups of genes responded in partially overlapping waves. These correspond roughly to two quick waves of inflammation and cell proliferation, a slow wave of tissue remodeling and debris removal, and a final stationary phase that primarily reflects permanent structural changes in the axons. Responses from astrocytes, microglia and oligodendrocytes were distinctively different, both molecularly and morphologically. Comparisons to other models of brain injury and to glaucoma indicated that the glial responses depended on both the tissue and the injury. Attempts to modulate glial function after axonal injuries should consider different mechanistic targets at different times following the insult.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

