The putative protein methyltransferase LAE1 of Trichoderma atroviride is a key regulator of asexual development and mycoparasitism
- PMID: 23826217
- PMCID: PMC3691206
- DOI: 10.1371/journal.pone.0067144
The putative protein methyltransferase LAE1 of Trichoderma atroviride is a key regulator of asexual development and mycoparasitism
Abstract
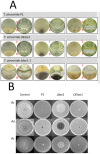
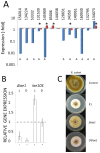
In Ascomycota the protein methyltransferase LaeA is a global regulator that affects the expression of secondary metabolite gene clusters, and controls sexual and asexual development. The common mycoparasitic fungus Trichoderma atroviride is one of the most widely studied agents of biological control of plant-pathogenic fungi that also serves as a model for the research on regulation of asexual sporulation (conidiation) by environmental stimuli such as light and/or mechanical injury. In order to learn the possible involvement of LAE1 in these two traits, we assessed the effect of deletion and overexpression of lae1 gene on conidiation and mycoparasitic interaction. In the presence of light, conidiation was 50% decreased in a Δ lae1 and 30-50% increased in lae1-overexpressing (OElae1) strains. In darkness, Δ lae1 strains did not sporulate, and the OElae1 strains produced as much spores as the parent strain. Loss-of-function of lae1 also abolished sporulation triggered by mechanical injury of the mycelia. Deletion of lae1 also increased the sensitivity of T. atroviride to oxidative stress, abolished its ability to defend against other fungi and led to a loss of mycoparasitic behaviour, whereas the OElae1 strains displayed enhanced mycoparasitic vigor. The loss of mycoparasitic activity in the Δ lae1 strain correlated with a significant underexpressionn of several genes normally upregulated during mycoparasitic interaction (proteases, GH16 ß-glucanases, polyketide synthases and small cystein-rich secreted proteins), which in turn was reflected in the partial reduction of formation of fungicidal water soluble metabolites and volatile compounds. Our study shows T. atroviride LAE1 is essential for asexual reproduction in the dark and for defense and parasitism on other fungi.
Conflict of interest statement
Figures



References
-
- Druzhinina IS, Seidl-Seiboth V, Herrera-Estrella A, Horwitz BA, Kenerley CM, et al. (2011) Trichoderma: the genomics of opportunistic success. Nature Reviews Microbiology 16: 749–759. - PubMed
-
- Verma M, Brar SK, Tyagi RD, Surampalli RY, Val'ero RJ (2007) Antagonistic fungi, Trichoderma spp.: panoply of biological control. Biochem Eng. J. 37: 1–20.
-
- Harman GE, Howell CR, Viterbo A, Chet I, Lorito M (2004) Trichoderma species - opportunistic, avirulent plant symbionts. Nature Reviews Microbiology 2: 43–56. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

