BrainAGE in Mild Cognitive Impaired Patients: Predicting the Conversion to Alzheimer's Disease
- PMID: 23826273
- PMCID: PMC3695013
- DOI: 10.1371/journal.pone.0067346
BrainAGE in Mild Cognitive Impaired Patients: Predicting the Conversion to Alzheimer's Disease
Abstract
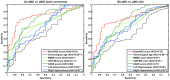
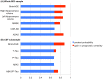
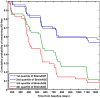
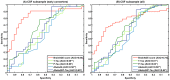
Alzheimer's disease (AD), the most common form of dementia, shares many aspects of abnormal brain aging. We present a novel magnetic resonance imaging (MRI)-based biomarker that predicts the individual progression of mild cognitive impairment (MCI) to AD on the basis of pathological brain aging patterns. By employing kernel regression methods, the expression of normal brain-aging patterns forms the basis to estimate the brain age of a given new subject. If the estimated age is higher than the chronological age, a positive brain age gap estimation (BrainAGE) score indicates accelerated atrophy and is considered a risk factor for conversion to AD. Here, the BrainAGE framework was applied to predict the individual brain ages of 195 subjects with MCI at baseline, of which a total of 133 developed AD during 36 months of follow-up (corresponding to a pre-test probability of 68%). The ability of the BrainAGE framework to correctly identify MCI-converters was compared with the performance of commonly used cognitive scales, hippocampus volume, and state-of-the-art biomarkers derived from cerebrospinal fluid (CSF). With accuracy rates of up to 81%, BrainAGE outperformed all cognitive scales and CSF biomarkers in predicting conversion of MCI to AD within 3 years of follow-up. Each additional year in the BrainAGE score was associated with a 10% greater risk of developing AD (hazard rate: 1.10 [CI: 1.07-1.13]). Furthermore, the post-test probability was increased to 90% when using baseline BrainAGE scores to predict conversion to AD. The presented framework allows an accurate prediction even with multicenter data. Its fast and fully automated nature facilitates the integration into the clinical workflow. It can be exploited as a tool for screening as well as for monitoring treatment options.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

