The role of glycoprotein 130 family of cytokines in fetal rat lung development
- PMID: 23826327
- PMCID: PMC3691159
- DOI: 10.1371/journal.pone.0067607
The role of glycoprotein 130 family of cytokines in fetal rat lung development
Abstract
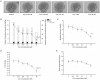
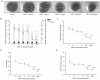
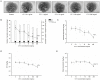
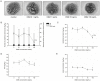
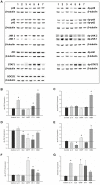
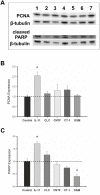
The glycoprotein 130 (gp130) dependent family of cytokines comprises interleukin-6 (IL-6), IL-11, leukemia inhibitory factor (LIF), cardiotrophin-like cytokine (CLC), ciliary neurotrophic factor (CNTF), cardiotrophin-1 (CT-1) and oncostatin M (OSM). These cytokines share the membrane gp130 as a common signal transducer. Recently, it was demonstrated that IL-6 promotes, whereas LIF inhibits fetal lung branching. Thus, in this study, the effects on fetal lung morphogenesis of the other classical members of the gp130-type cytokines (IL-11, CLC, CNTF, CT-1 and OSM) were investigated. We also provide the first description of these cytokines and their common gp130 receptor protein expression patterns during rat lung development. Fetal rat lung explants were cultured in vitro with increasing concentrations of IL-11, CLC, CNTF, CT-1 and OSM. Treated lung explants were morphometrically analyzed and assessed for MAPK, PI3K/AKT and STAT3 signaling modifications. IL-11, which similarly to IL-6 acts through a gp130 homodimer receptor, significantly stimulated lung growth via p38 phosphorylation. On the other hand, CLC, CNTF, CT-1 and OSM, whose receptors are gp130 heterodimers, inhibited lung growth acting in different signal-transducing pathways. Thus, the present study demonstrated that although cytokines of the gp130 family share a common signal transducer, there are specific biological activities for each cytokine on lung development. Indeed, cytokine signaling through gp130 homodimers stimulate, whereas cytokine signaling through gp130 heterodimers inhibit lung branching.
Conflict of interest statement
Figures
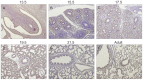
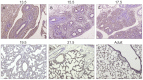

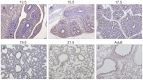


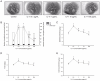
Similar articles
-
gp130 Cytokines Activate Novel Signaling Pathways and Alter Bone Dissemination in ER+ Breast Cancer Cells.J Bone Miner Res. 2022 Feb;37(2):185-201. doi: 10.1002/jbmr.4430. Epub 2021 Sep 17. J Bone Miner Res. 2022. PMID: 34477239 Free PMC article.
-
The cytokines cardiotrophin-like cytokine/cytokine-like factor-1 (CLC/CLF) and ciliary neurotrophic factor (CNTF) differ in their receptor specificities.Cytokine. 2012 Dec;60(3):653-60. doi: 10.1016/j.cyto.2012.08.014. Epub 2012 Sep 15. Cytokine. 2012. PMID: 22986012
-
Interleukin-6 family of cytokines induced activation of different functional sites expressed by gp130 transducing protein.J Biol Chem. 1996 Jun 21;271(25):14764-72. doi: 10.1074/jbc.271.25.14764. J Biol Chem. 1996. PMID: 8662918
-
Conditional gp130 deficient mouse mutants.Semin Cell Dev Biol. 2008 Aug;19(4):379-84. doi: 10.1016/j.semcdb.2008.07.001. Epub 2008 Jul 17. Semin Cell Dev Biol. 2008. PMID: 18687405 Review.
-
The cytokine receptor gp130: faithfully promiscuous.Sci STKE. 2003 Sep 23;2003(201):PE40. doi: 10.1126/stke.2003.201.pe40. Sci STKE. 2003. PMID: 14506288 Review.
Cited by
-
Neuroendocrine factors regulate retinoic acid receptors in normal and hypoplastic lung development.J Physiol. 2015 Aug 1;593(15):3301-11. doi: 10.1113/JP270477. Epub 2015 Jul 14. J Physiol. 2015. PMID: 26096456 Free PMC article.
-
Secreted phosphoprotein 1 is a determinant of lung function development in mice.Am J Respir Cell Mol Biol. 2014 Nov;51(5):637-51. doi: 10.1165/rcmb.2013-0471OC. Am J Respir Cell Mol Biol. 2014. PMID: 24816281 Free PMC article.
-
Research progress on S-palmitoylation modification mediated by the ZDHHC family in glioblastoma.Front Cell Dev Biol. 2024 Nov 5;12:1413708. doi: 10.3389/fcell.2024.1413708. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39563863 Free PMC article. Review.
-
Interleukin-11 Is Involved in Hyperoxia-induced Bronchopulmonary Dysplasia in Newborn Mice by Mediating Epithelium-Fibroblast Cross-talk.Inflammation. 2025 Apr;48(2):796-805. doi: 10.1007/s10753-024-02089-0. Epub 2024 Jul 24. Inflammation. 2025. PMID: 39046604
-
Distinct Epithelial Cell Profiles in Normal Versus Induced-Congenital Diaphragmatic Hernia Fetal Lungs.Front Pediatr. 2022 May 6;10:836591. doi: 10.3389/fped.2022.836591. eCollection 2022. Front Pediatr. 2022. PMID: 35601428 Free PMC article.
References
-
- Warburton D, Bellusci S, De Langhe S, Del Moral PM, Fleury V, et al. (2005) Molecular mechanisms of early lung specification and branching morphogenesis. Pediatr Res 57: 26R–37R. - PubMed
-
- Nogueira-Silva C, Santos M, Baptista MJ, Moura RS, Correia-Pinto J (2006) IL-6 is constitutively expressed during lung morphogenesis and enhances fetal lung explant branching. Pediatr Res 60: 530–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

