Dapper antagonist of catenin-1 cooperates with Dishevelled-1 during postsynaptic development in mouse forebrain GABAergic interneurons
- PMID: 23826333
- PMCID: PMC3691262
- DOI: 10.1371/journal.pone.0067679
Dapper antagonist of catenin-1 cooperates with Dishevelled-1 during postsynaptic development in mouse forebrain GABAergic interneurons
Abstract
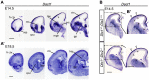
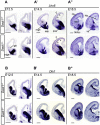
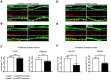
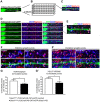
Synaptogenesis has been extensively studied along with dendritic spine development in glutamatergic pyramidal neurons, however synapse development in cortical interneurons, which are largely aspiny, is comparatively less well understood. Dact1, one of 3 paralogous Dact (Dapper/Frodo) family members in mammals, is a scaffold protein implicated in both the Wnt/β-catenin and the Wnt/Planar Cell Polarity pathways. We show here that Dact1 is expressed in immature cortical interneurons. Although Dact1 is first expressed in interneuron precursors during proliferative and migratory stages, constitutive Dact1 mutant mice have no major defects in numbers or migration of these neurons. However, cultured cortical interneurons derived from these mice have reduced numbers of excitatory synapses on their dendrites. We selectively eliminated Dact1 from mouse cortical interneurons using a conditional knock-out strategy with a Dlx-I12b enhancer-Cre allele, and thereby demonstrate a cell-autonomous role for Dact1 during postsynaptic development. Confirming this cell-autonomous role, we show that synapse numbers in Dact1 deficient cortical interneurons are rescued by virally-mediated re-expression of Dact1 specifically targeted to these cells. Synapse numbers in these neurons are also rescued by similarly targeted expression of the Dact1 binding partner Dishevelled-1, and partially rescued by expression of Disrupted in Schizophrenia-1, a synaptic protein genetically implicated in susceptibility to several major mental illnesses. In sum, our results support a novel cell-autonomous postsynaptic role for Dact1, in cooperation with Dishevelled-1 and possibly Disrupted in Schizophrenia-1, in the formation of synapses on cortical interneuron dendrites.
Conflict of interest statement
Figures

References
-
- Badawy RA, Harvey AS, Macdonell RA (2009) Cortical hyperexcitability and epileptogenesis: Understanding the mechanisms of epilepsy - part 2. J Clin Neurosci 16: 485–500. - PubMed
-
- Benes FM, Berretta S (2001) GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology 25: 1–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

